Specific Enrichment of Hyperthermophilic Electroactive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Resolved Meta-Analysis of the Microbiome in Oil Reservoirs Worldwide
microorganisms Article Genome-Resolved Meta-Analysis of the Microbiome in Oil Reservoirs Worldwide Kelly J. Hidalgo 1,2,* , Isabel N. Sierra-Garcia 3 , German Zafra 4 and Valéria M. de Oliveira 1 1 Microbial Resources Division, Research Center for Chemistry, Biology and Agriculture (CPQBA), University of Campinas–UNICAMP, Av. Alexandre Cazellato 999, 13148-218 Paulínia, Brazil; [email protected] 2 Graduate Program in Genetics and Molecular Biology, Institute of Biology, University of Campinas (UNICAMP), Rua Monteiro Lobato 255, Cidade Universitária, 13083-862 Campinas, Brazil 3 Biology Department & CESAM, University of Aveiro, Aveiro, Portugal, Campus de Santiago, Avenida João Jacinto de Magalhães, 3810-193 Aveiro, Portugal; [email protected] 4 Grupo de Investigación en Bioquímica y Microbiología (GIBIM), Escuela de Microbiología, Universidad Industrial de Santander, Cra 27 calle 9, 680002 Bucaramanga, Colombia; [email protected] * Correspondence: [email protected]; Tel.: +55-19981721510 Abstract: Microorganisms inhabiting subsurface petroleum reservoirs are key players in biochemical transformations. The interactions of microbial communities in these environments are highly complex and still poorly understood. This work aimed to assess publicly available metagenomes from oil reservoirs and implement a robust pipeline of genome-resolved metagenomics to decipher metabolic and taxonomic profiles of petroleum reservoirs worldwide. Analysis of 301.2 Gb of metagenomic information derived from heavily flooded petroleum reservoirs in China and Alaska to non-flooded petroleum reservoirs in Brazil enabled us to reconstruct 148 metagenome-assembled genomes (MAGs) of high and medium quality. At the phylum level, 74% of MAGs belonged to bacteria and 26% to archaea. The profiles of these MAGs were related to the physicochemical parameters and recovery management applied. -

Supplementary Materials
SUPPLEMENTARY MATERIALS Table S1. Chemical characteristics of the two digestate forms (SD and WD). Values quoted are expressed as % of air-dry digestate (means followed by standard error in brackets). References for the employed methods used for determination of each chemical characteristic is also reported. SD WD Reference Org C % 44.4 (0.33) 1.1 (0.01) [81] Tot N % 1.4 (0.01) 0.4 (0.01) [82] C/N 31.4 (0.17) 3.1 (0.04) NH4-N % n.d 0.2 (0.00) [83] K % 1.7 (0.00) n.d. [84] P % 0.9 (0.01) n.d. [84] S % 0.23 (0.02) n.d. [85] SD = solid digestate; WD = whole digestate. Table S2. Soil physical and chemical characteristics at the beginning of trial (t0) (means from 9 observations followed by standard errors in brackets). Clay (%) 41.9 (1.22) Silt (%) 47.8 (2.13) Moisture (%) 24.46 (1.24) Bulk density (g cm-3) 1.39 (0.04) pH 8.3 (0) CaCO3 (%) 11.4 (0.7) TOC (g kg-1) 12.8 (0.3) TN (g kg-1) 1.4 (0) C/N 9.4 (0.3) CEC (cmol(+) kg-1) 21.0 (0.7) Exchangeable Bases (mg kg-1) K 278.7 (8.0) Na 22.0 (3.0) Mg 201.7 (25.6) Ca 3718.6 (176.3) Available Microelements (mg kg-1) Cu 28.0 (5.0) Zn 1.7 (0.2) Fe 15.4 (0.5) Mn 16.3 (0.5) TOC = total organic C; TN = total N; CEC = cation exchange capacity Table S3. -

Archaeoglobus Profundus Type Strain (AV18T)
Standards in Genomic Sciences (2010) 2:327-346 DOI:10.4056/sigs.942153 Complete genome sequence of Archaeoglobus profundus type strain (AV18T) Mathias von Jan1, Alla Lapidus2, Tijana Glavina Del Rio2, Alex Copeland2, Hope Tice2, Jan-Fang Cheng2, Susan Lucas2, Feng Chen2, Matt Nolan2, Lynne Goodwin2,3, Cliff Han2,3, Sam Pitluck2, Konstantinos Liolios2, Natalia Ivanova2, Konstantinos Mavromatis2, Galina Ovchinnikova2, Olga Chertkov2, Amrita Pati2, Amy Chen4, Krishna Palaniappan4, Miriam Land2,5, Loren Hauser2,5, Yun-Juan Chang2,5, Cynthia D. Jeffries2,5, Elizabeth Saunders2, Thomas Brettin2,3, John C. Detter2,3, Patrick Chain2,4, Konrad Eichinger6, Harald Huber6, Ste- fan Spring1, Manfred Rohde7, Markus Göker1, Reinhard Wirth6, Tanja Woyke2, Jim Bristow2, Jonathan A. Eisen2,8, Victor Markowitz4, Philip Hugenholtz2, Nikos C Kyrpides2, and Hans-Peter Klenk1* 1 DSMZ - German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany 2 DOE Joint Genome Institute, Walnut Creek, California, USA 3 Los Alamos National Laboratory, Bioscience Division, Los Alamos, New Mexico, USA 4 Biological Data Management and Technology Center, Lawrence Berkeley National Laboratory, Berkeley, California, USA 5 Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA 6 University of Regensburg, Microbiology – Archaeenzentrum, Regensburg, Germany 7 HZI – Helmholtz Centre for Infection Research, Braunschweig, Germany 8 University of California Davis Genome Center, Davis, California, USA *Corresponding author: Hans-Peter Klenk Keywords: hyperthermophilic, marine, strictly anaerobic, sulfate respiration, hydrogen utili- zation, hydrothermal systems, Archaeoglobaceae, GEBA Archaeoglobus profundus (Burggraf et al. 1990) is a hyperthermophilic archaeon in the eu- ryarchaeal class Archaeoglobi, which is currently represented by the single family Archaeog- lobaceae, containing six validly named species and two strains ascribed to the genus 'Geoglobus' which is taxonomically challenged as the corresponding type species has no va- lidly published name. -

Bacterial Profiles and Antibiogrants of the Bacteria Isolated of the Exposed Pulps of Dog and Cheetah Canine Teeth
Bacterial profiles and antibiogrants of the bacteria isolated of the exposed pulps of dog and cheetah canine teeth A dissertation submitted to the Faculty of Veterinary Science, University of Pretoria. In partial fulfillment of the requirements for the degree Master of Science (Veterinary Science) Promoter: Dr. Gerhard Steenkamp Co-promoter: Ms. Anna-Mari Bosman Department of Companion Animal Clinical Studies Faculty of Veterinary Science University of Pretoria Pretoria (January 2012) J.C. Almansa Ruiz © University of Pretoria Declaration I declare that the dissertation that I hereby submit for the Masters of Science degree in Veterinary Science at the University of Pretoria has not previously been submitted by me for degree purposes at any other university. J.C. Almansa Ruiz ii Dedications To one of the most amazing hunters of the African bush, the Cheetah, that has made me dream since I was a child, and the closest I had been to one, before starting this project was in National Geographic documentaries. I wish all of them a better future in which their habitat will be more respected. To all conservationists, especially to Carla Conradie and Dave Houghton, for spending their lives saving these animals which are suffering from the consequences of the encroachment of human beings into their territory. Some, such as George Adamson, the lion conservationist, even lost their lives in this mission. To the conservationist, Lawrence Anthony, for risking his life in a suicide mission to save the animals in the Baghdad Zoo, when the conflict in Iraq exploded. To my mother and father Jose Maria and Rosa. -

Specific Enrichment of Hyperthermophilic Electroactive Archaea From
bioRxiv preprint doi: https://doi.org/10.1101/272039; this version posted February 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Specific enrichment of hyperthermophilic electroactive Archaea from 2 deep-sea hydrothermal vent on electrically conductive support. 3 4 Guillaume Pillot1, Eléonore Frouin1, Emilie Pasero1, Anne Godfroy2, Yannick Combet-Blanc1, 5 Sylvain Davidson1 & Pierre-Pol Liebgott1* 6 1 Aix Marseille Université, IRD, Université de Toulon, CNRS, MIO UM 110, Marseille, France. 7 2 Laboratoire de Microbiologie des Environnements Extrêmes - UMR6197 IFREMER, CNRS, 8 UBO Centre de Brest–CS10070/IUEM, Plouzané, France. 9 *Corresponding author: [email protected]; Mediterranean Institute of 10 Oceanography, Campus de Luminy, Bâtiment OCEANOMED, 13288 Marseille Cedex 09. 11 Abstract 12 While more and more investigations are done to isolate hyperthermophilic exoelectrogenic 13 communities from environments, none have been performed yet on deep-sea hydrothermal vent. 14 Samples of black smoker chimney from Rainbow site on the Atlantic mid-oceanic ridge have 15 been harvested for enriching exoelectrogens in microbial electrolysis cells under hyperthermophilic 16 (80°C) condition. Two enrichments have been performed: one from direct inoculation of crushed 17 chimney and the other one from inoculation of a pre-cultivation on iron (III) oxide. In both 18 experiments, a current production was observed from 2.4 A/m² to 5.8 A/m² with a set anode 19 potential of +0.05 vs SHE. Taxonomic affiliation of the exoelectrogen communities obtained 20 exhibited a specific enrichment of Archaea from Thermococcales and Archeoglobales orders on the 21 electrode, even when both inocula were dominated by Bacteria. -

United States Patent (10) Patent No.: US 7820,184 B2 Stritzker Et Al
USOO782O184B2 (12) United States Patent (10) Patent No.: US 7820,184 B2 Stritzker et al. (45) Date of Patent: Oct. 26, 2010 (54) METHODS AND COMPOSITIONS FOR 5,833,975 A 1 1/1998 Paoletti et al. ............. 424.93.2 DETECTION OF MICROORGANISMS AND 5,976,796. A 1 1/1999 Szalay et al. ................... 435/6 SirNRTREATMENT OF DISEASES AND 6,025,155 A 2/2000 Hadlaczky et al. ......... 435/69.1 6,045,802 A 4/2000 Schlom et al. ........... 424,199.1 (75) Inventors: Jochen Harald Stritzker, Kissing (DE); 6,077,697 A 6/2000 Hadlaczky et al. 435/1723 Phil Hill, West Bridgford (GB); Aladar 6,080,849 A 6/2000 Bermudes et al. .......... 536,23.7 A. Szalay, Highland, CA (US); Yong A. 6,093,700 A 7/2000 Mastrangelo et al. ......... 514,44 Yu, San Diego, CA (US) 6,099,848. A 8/2000 Frankel et al. ........... 424,246.1 6,106,826 A 8/2000 Brandt et al. .............. 424.93.2 (73) Assignee: stylus Corporation, San Diego, CA 6, 190,657 B1 2/2001 Pawelek et al. ............ 424,931 6,217,847 B1 4/2001 Contaget al. ................ 4249.1 (*) Notice: Subject to any disclaimer, the term of this 6,232,523 B1 5/2001 Tan et al. ...................... 800, 10 patent is extended or adjusted under 35 6,235,967 B1 5/2001 Tan et al. ...................... 800, 10 U.S.C. 154(b) by 362 days. 6,235,968 B1 5/2001 Tan et al. ...................... 800, 10 6,251,384 B1 6/2001 Tan et al. -
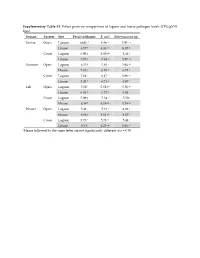
Supplementary Table S1. Fisher Pairwise Comparisons of Lagoon and House Pathogen Levels (CFU/Gvss Log10) Season System Site Fecal Coliforms E
Supplementary Table S1. Fisher pairwise comparisons of lagoon and house pathogen levels (CFU/gVSS log10) Season System Site Fecal coliforms E. coli Enterococcus sp. Spring Open Lagoon 6.00 g1 5.46 hi 5.91 cd House 6.57 e 6.33 cd 6.35 b Cover Lagoon 6.00 g 5.60 gh 5.44 f House 7.73 a 7.63 a 5.87 cd Summer Open Lagoon 6.35 f 5.83 f 5.94 cd House 7.83 a 6.10 e 6.73 a Cover Lagoon 7.04 c 6.47 c 5.86 cd House 7.42 b 6.71 b 6.07 c Fall Open Lagoon 5.58 i 5.58 gh 5.56 ef House 6.81 d 6.72 b 6.01 c Cover Lagoon 5.99 g 5.34 i 5.50 f House 6.38 f 6.19 de 5.74 de Winter Open Lagoon 5.41 j 5.13 j 4.88 g House 6.06 g 5.61 gh 6.67 a Cover Lagoon 5.73 h 5.73 fg 5.44 f House 6.34 f 6.25 de 5.86 cd 1Means followed by the same letter are not significantly different at p = 0.05 Supplementary Table S2. Relative abundances of OTUs identified using universal bacterial primer set, presented as relative abundances (%) Only bacterial families are counted in Figure 5 and discussion involving bacterial family identification. # Identified in # Identified from all 8 All 16 All #OTU Table SpOLRA SuOLRA FOLRA WOLRA SpCLRA SuCLRA FCLRA WCLRA SpOHRA SuOHRA FOHRA WOHRA SpCHRA SuCHRA FCHRA WCHRA all samples lagoon samples Samples Lagoons Notes Key Patulibacteraceae 0 9.93739E‐05* 0.000134 0.000116 0 0 0 0 0 0 0.000452 0 0 0 0 0 4 3 Sp = Spring O = Open Ruaniaceae 0 0 0 0 0 0 0 0 0 0 0 0 0 0.000221577 0 7.73E‐05 2 0 Su = Summer C = Covered Thermoactinomycetaceae 0 0 0.000267 0.000116 0 0.00037092 0.000529 0.000526 0 0.000342922 0.000271 0.000197 0 0 0 0 8 5 F = Fall L = Lagoon Beutenbergiaceae 0.000226334 0.000331246 0.000579 0.001663 0.000620176 0 0.000106 0.000807 0.002320743 0.000571537 0.000723 0.000591 0.000169731 0 0.000606 0 13 7 W = Winter H = House Thermoanaerobacterales Family III. -

Proteiniborus Ethanoligenes Gen. Nov., Sp. Nov., an Anaerobic Protein-Utilizing Bacterium
%paper no. ije65108 charlesworth ref: ije65108& New Taxa - Other Gram-positive Bacteria International Journal of Systematic and Evolutionary Microbiology (2008), 58, 000–000 DOI 10.1099/ijs.0.65108-0 Proteiniborus ethanoligenes gen. nov., sp. nov., an anaerobic protein-utilizing bacterium Lili Niu,1,2 Lei Song1,2 and Xiuzhu Dong1 Correspondence 1State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Xiuzhu Dong Sciences, Beijing 100101, PR China [email protected] 2Graduate School, Chinese Academy of Sciences, Beijing 100049, PR China A novel anaerobic, mesophilic, protein-utilizing bacterial strain, GWT, was isolated from the mesophilic hydrogen-producing granular sludge used to treat food industry wastewater. The strain was a Gram-positive, non-spore-forming and non-motile rod. Growth of the strain was observed at 20–48 6C and at pH 6.4–10.0. The strain used yeast extract and peptone as carbon and energy sources. Weak growth was also observed with tryptone and Casamino acids as carbon and energy sources. The strain used none of the tested carbohydrates, alcohols or fatty acids. The fermentation products in peptone-yeast broth included ethanol, acetic acid, hydrogen and carbon dioxide. Gelatin was not hydrolysed. Nitrate was reduced. Indole was produced. T NH3 and H2S were not produced. The DNA G+C content of strain GW was 38.0 mol%. The predominant cellular fatty acids were the saturated fatty acids C14 : 0 (15.58 %), C16 : 0 (25.40 %) and C18 : 0 (12.03 %). Phylogenetic analysis based on 16S rRNA gene sequence similarity revealed that strain GWT represented a new branch within cluster XII of the Clostridium subphylum, with ,89.6 % 16S rRNA gene sequence similarities to all described species. -

Electrical Current Generation in Microbial Electrolysis Cells by Hyperthermophilic Archaea Ferroglobus Placidus and Geoglobus Ahangari
Bioelectrochemistry 119 (2018) 142–149 Contents lists available at ScienceDirect Bioelectrochemistry journal homepage: www.elsevier.com/locate/bioelechem Electrical current generation in microbial electrolysis cells by hyperthermophilic archaea Ferroglobus placidus and Geoglobus ahangari Yasemin D. Yilmazel a,b,⁎, Xiuping Zhu b,c,Kyoung-YeolKimb,DawnE.Holmesd, Bruce E. Logan b a Department of Chemical Engineering, Rochester Institute of Technology, Rochester, NY, USA b Department of Civil and Environmental Engineering, The Pennsylvania State University, University Park, PA, USA c Department of Civil and Environmental Engineering, Louisiana State University, Baton Rouge, LA, USA d Department of Biology, Western New England University, Springfield, MA, USA article info abstract Article history: Few microorganisms have been examined for current generation under thermophilic (40–65 °C) or hyperther- Received 5 May 2017 mophilic temperatures (≥80 °C) in microbial electrochemical systems. Two iron-reducing archaea from the fam- Received in revised form 27 September 2017 ily Archaeoglobaceae, Ferroglobus placidus and Geoglobus ahangari, showed electro-active behavior leading to Accepted 28 September 2017 current generation at hyperthermophilic temperatures in single-chamber microbial electrolysis cells (MECs). A Available online 02 October 2017 current density (j) of 0.68 ± 0.11 A/m2 was attained in F. placidus MECs at 85 °C, and 0.57 ± 0.10 A/m2 in G. ahangari MECs at 80 °C, with an applied voltage of 0.7 V. Cyclic voltammetry (CV) showed that both strains pro- Keywords: − − Hyperthermophilic archaea duced a sigmoidal catalytic wave, with a mid-point potential of 0.39 V (vs. Ag/AgCl) for F. placidus and 0.37 V Ferroglobus placidus for G. -

Divergent Methyl-Coenzyme M Reductase Genes in a Deep-Subseafloor Archaeoglobi
The ISME Journal (2019) 13:1269–1279 https://doi.org/10.1038/s41396-018-0343-2 ARTICLE Divergent methyl-coenzyme M reductase genes in a deep-subseafloor Archaeoglobi 1 2,3 1 1 1 4 Joel A. Boyd ● Sean P. Jungbluth ● Andy O. Leu ● Paul N. Evans ● Ben J. Woodcroft ● Grayson L. Chadwick ● 4 5 6 1 Victoria J. Orphan ● Jan P. Amend ● Michael S. Rappé ● Gene W. Tyson Received: 7 October 2018 / Revised: 29 November 2018 / Accepted: 11 December 2018 / Published online: 16 January 2019 © The Author(s) 2019. This article is published with open access Abstract The methyl-coenzyme M reductase (MCR) complex is a key enzyme in archaeal methane generation and has recently been proposed to also be involved in the oxidation of short-chain hydrocarbons including methane, butane, and potentially propane. The number of archaeal clades encoding the MCR continues to grow, suggesting that this complex was inherited from an ancient ancestor, or has undergone extensive horizontal gene transfer. Expanding the representation of MCR- encoding lineages through metagenomic approaches will help resolve the evolutionary history of this complex. Here, a near- complete Archaeoglobi metagenome-assembled genome (MAG; Ca. Polytropus marinifundus gen. nov. sp. nov.) was fl fl 1234567890();,: 1234567890();,: recovered from the deep subsea oor along the Juan de Fuca Ridge ank that encodes two divergent McrABG operons similar to those found in Ca. Bathyarchaeota and Ca. Syntrophoarchaeum MAGs. Ca. P. marinifundus is basal to members of the class Archaeoglobi, and encodes the genes for β-oxidation, potentially allowing an alkanotrophic metabolism similar to that proposed for Ca. -

( 12 ) United States Patent
US010398154B2 (12 ) United States Patent ( 10 ) Patent No. : US 10 , 398 , 154 B2 Embree et al. ( 45 ) Date of Patent: * Sep . 3 , 2019 (54 ) MICROBIAL COMPOSITIONS AND ( 58 ) Field of Classification Search METHODS OF USE FOR IMPROVING MILK None PRODUCTION See application file for complete search history . (71 ) Applicant: ASCUS BIOSCIENCES , INC ., San Diego , CA (US ) (56 ) References Cited ( 72 ) Inventors: Mallory Embree, San Diego , CA (US ) ; U . S . PATENT DOCUMENTS Luke Picking , San Diego , CA ( US ) ; 3 , 484 , 243 A 12 / 1969 Anderson et al . Grant Gogul , Cardiff , CA (US ) ; Janna 4 ,559 , 298 A 12 / 1985 Fahy Tarasova , San Diego , CA (US ) (Continued ) (73 ) Assignee : Ascus Biosciences , Inc. , San Diego , FOREIGN PATENT DOCUMENTS CA (US ) CN 104814278 A 8 / 2015 ( * ) Notice : Subject to any disclaimer , the term of this EP 0553444 B1 3 / 1998 patent is extended or adjusted under 35 U .S . C . 154 (b ) by 0 days. (Continued ) This patent is subject to a terminal dis OTHER PUBLICATIONS claimer . Borling , J , Master' s thesis, 2010 . * (21 ) Appl. No .: 16 / 029, 398 ( Continued ) ( 22 ) Filed : Jul. 6 , 2018 Primary Examiner - David W Berke- Schlessel (65 ) Prior Publication Data ( 74 ) Attorney , Agent, or Firm — Cooley LLP US 2018 /0325966 A1 Nov . 15 , 2018 ( 57 ) ABSTRACT Related U . S . Application Data The disclosure relates to isolated microorganisms — includ (63 ) Continuation of application No. ing novel strains of the microorganisms - microbial consor PCT/ US2014 /012573 , filed on Jan . 6 , 2017 . tia , and compositions comprising the same . Furthermore , the disclosure teaches methods of utilizing the described micro (Continued ) organisms, microbial consortia , and compositions compris (51 ) Int. -

Hydrogen Stress and Syntrophy of Hyperthermophilic Heterotrophs and Methanogens
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses July 2018 HYDROGEN STRESS AND SYNTROPHY OF HYPERTHERMOPHILIC HETEROTROPHS AND METHANOGENS Begum Topcuoglu University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Environmental Microbiology and Microbial Ecology Commons, and the Microbial Physiology Commons Recommended Citation Topcuoglu, Begum, "HYDROGEN STRESS AND SYNTROPHY OF HYPERTHERMOPHILIC HETEROTROPHS AND METHANOGENS" (2018). Doctoral Dissertations. 1299. https://doi.org/10.7275/11912692.0 https://scholarworks.umass.edu/dissertations_2/1299 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. HYDROGEN STRESS AND SYNTROPHY OF HYPERTHERMOPHILIC HETEROTROPHS AND METHANOGENS A Dissertation Presented by BEGÜM D. TOPÇUOĞLU Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY May 2018 Microbiology © Copyright by Begüm Topçuoğlu 2018 All Rights Reserved HYDROGEN STRESS AND SYNTROPHY OF HYPERTHERMOPHILIC HETEROTROPHS AND METHANOGENS A Dissertation Presented by BEGÜM D. TOPÇUOĞLU Approved as to style and content by: ____________________________________