QUILLAIA EXTRACTS Type 1 and Type 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
GRAS Notice (GRN) No. 903 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory NATUREX• part of Givaudan 'fR1@CG~nill~(jJ) 07 January 2020 JAN 1 5 2020 Dr. Paulette Gaynor FOOD OF, ,Li,; UF L ADDITIVE SAFETY Office of Food Additive Safety (HFS-200) __J Center for Food Safety and Applied Nutrition (CFSAN) Food and Drug Administration 5001 Campus Drive College Park, MD 20740 USA Dear Dr. Gaynor: Re: GRAS Notice for Qulllaia Extract Type 2 In accordance with 21 CFR §170 Subpart E consisting of§§ 170.203 through 170.285, Naturex SA (250 rue Pierre Bayle, BP 81218 - 84911, Avignon, Cedex 9, France], as the notifier, is submitting one hard copy and one electronic copy (on CD) of the GRAS Notice for quillaia extract type 2, containing all data and lnfonnation supporting the company's conclusion that quillaia extract type 2 is GRAS on the basis of scientific procedures, for use in specified conventional food and beverage products across multiple categories; these food uses of quillaia extract type 2 are therefore not subject to the premarket approval requirements of the Federal Food, Drug and Cosmetic Act. Information setting forth the basis for Naturex's GRAS conclusion, as well as a consensus opinion of an independent panel of experts, also are enclosed for review by the agency. I certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using Symantec Endpoint Protection 12.1.4. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. -
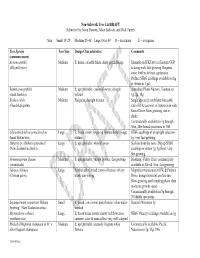
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

The Biological Action of Saponins in Animal Systems: a Review
Downloaded from https://www.cambridge.org/core British Journal of Nutrition (2002), 88, 587–605 DOI: 10.1079/BJN2002725 q The Authors 2002 The biological action of saponins in animal systems: a review . IP address: 1 2 3 1 George Francis , Zohar Kerem , Harinder P. S. Makkar and Klaus Becker * 170.106.202.58 1Department of Aquaculture Systems and Animal Nutrition, Institute for Animal Production in the Tropics and Subtropics, University of Hohenheim (480), D 70593 Stuttgart, Germany 2Institute of Biochemistry, Food Science and Nutrition, Faculty of Agricultural, Food and Environmental Quality Sciences, , on The Hebrew University of Jerusalem, P.O.B. 12, Rehovot 76100, Israel 3Animal Production and Health Section, International Atomic Energy Agency, P.O. Box 100, Wagramerstr. 5, A-1400 29 Sep 2021 at 04:10:30 Vienna, Austria (Received 4 December 2001 – Revised 19 June 2002 – Accepted 11 August 2002) , subject to the Cambridge Core terms of use, available at Saponins are steroid or triterpenoid glycosides, common in a large number of plants and plant products that are important in human and animal nutrition. Several biological effects have been ascribed to saponins. Extensive research has been carried out into the membrane-permeabilis- ing, immunostimulant, hypocholesterolaemic and anticarcinogenic properties of saponins and they have also been found to significantly affect growth, feed intake and reproduction in ani- mals. These structurally diverse compounds have also been observed to kill protozoans and molluscs, to be antioxidants, to impair the digestion of protein and the uptake of vitamins and minerals in the gut, to cause hypoglycaemia, and to act as antifungal and antiviral agents. -

Cyanogenic Glycosides • Isothiocyanate Glycosides • Phenolic & Aldehyde Glycosides • Coumarins - Bitter Principles • Terpenes and Terpenoids • Tannins Glycosides
Dr. Pran Kishore Deb and Dr. Yousef Abusamra Phytotherapy • Phytotherapy is the study of the use of extracts of natural origin as medicines or health-promoting agents. • Traditional phytotherapy is a synonym for herbalism and regarded as alternative medicine by much of Western medicine. PART – 1 • Glycosides • Saponin glycosides • Flavonoid glycosides • Anthocyanidin • Cyanogenic glycosides • Isothiocyanate glycosides • Phenolic & Aldehyde glycosides • Coumarins - Bitter principles • Terpenes and Terpenoids • Tannins Glycosides • They are compounds containing a carbohydrate (sugar) and a noncarbohydrate residue in the same molecule. • The carbohydrate residue is attached by an acetal linkage at carbon atom 1 to a noncarbohydrate residue. • The carbohydrate (sugar) component is called the GLYCONE. • The noncarbohydrate component is known as the AGLYCONE. • The sugar moiety can be joined to the aglycone in various ways: 1. Oxygen (O-glycoside) 2. Sulphur (S-glycoside) 3. Nitrogen (N-glycoside) 4. Carbon (C-glycoside) Classification of Glycosides • The aglycone may be methyl alcohol, glycerol, a sterol, a phenol, etc. • Based on the chemical nature of the aglycone group, glycosides can be classified as follows: 5 SAPONIN GLYCOSIDES SAPONIN GLYCOSIDES • Saponins on hydrolysis yield an aglycone known as "sapogenin". • Saponin glycosides are divided into 2 types based on the chemical structure of their aglycones (sapogenins). Classification of Saponins: According to the nature of the aglycone: 1. Neutral saponins or Steroidal saponins with spiroketal side chains. 2. Acid saponins or Triterpenoidal saponins Sug-O Sug-O Steroidal Saponins Triterpenoidal Saponins 7 Steroidal Saponins • Neutral steroidal glycosides which contain spiroketal side chain. • Two rings E and F called ketal because they are attached through two oxygen atoms and called spiral because they are not on the same level. -

Quillaja Saponaria (Molina) Extracts Inhibits in Vitro Piscirickettsia Salmonis Infections
animals Article Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections Hernán Cañon-Jones 1,* , Hernán Cortes 2, Mario Castillo-Ruiz 3,4 , Trinidad Schlotterbeck 5 and Ricardo San Martín 5 1 Núcleo de Investigación Aplicada en Ciencias Veterinarias y Agronómicas, Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago 7500975, Chile 2 Desert King Chile, Viña del Mar 2420505, Chile; [email protected] 3 Escuela de Química y Farmacia, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile; [email protected] 4 Departamento de Ciencias Químicas y Biológicas, Facultad de Ciencias de la Salud, Universidad Bernardo O Higgins, Santiago 8370993, Chile 5 Saponin Research Center, Santiago 7510132, Chile; [email protected] (T.S.); [email protected] (R.S.M.) * Correspondence: [email protected] Received: 15 September 2020; Accepted: 10 November 2020; Published: 3 December 2020 Simple Summary: Bacterial diseases causes massive mortalities in aquaculture and antibiotic use remains the main measure to keep these under control. Pisciricketssia salmonis, an intracellular bacterium only present in Chile, produces high mortalities in farmed salmon and is currently the main reason for using antimicrobials compared to other salmon-producing countries such as Norway. Environmental and antimicrobial resistance concerns have been raised by the local and global public and society, although no scientific evidence has demonstrated such an impact. Thus, there is a constant search for new alternatives that can complement or reduce the use of antimicrobial in intensive salmon farming. Phytochemicals such as saponins from Quillaja saponaria extracts have been proven to prevent and control diseases in other animal production systems. -

Histopathological and Toxicological Effects of Crude Saponin Extract from Phyllanthus Niruri, L (Syn
Global Journal of Medical research Volume 12 Issue 1 Version 1.0 February 2012 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4618 & Print ISSN : 0975-5888 Histopathological and Toxicological effects of crude saponin extract from Phyllanthus niruri, L (Syn. P. franternus. Webster) on Organs in animal studies By Ajibade V. A., Famurewa, O The Federal Polytechnic, P.M.B 5351, Ado-Ekiti, Nigeria Abstract – The histopathological view of liver, intestines and kidney of bacterial infected rabbits, fed with 100mg/ml saponin extracted from Phyllanthus niruri over a period of seven days was carried out to determine the effect of the plant extract on these organs after treatment. Saponin was administered as strawberry suspension at a dose of 10mg per day (divided into four doses) to ten rabbits, nine of which were fed with food contaminated with 0.5mL bacterial suspension obtained by McFarland standardization (10% Barium sulfate) after starvation for 6hrs . Multiple foci of tubular necrosis and haemorrhages in the kidney, marked hyperplasia of the mucosal layer of the small intestine, and a mild periportal lymphocytic cellular infiltration of the liver of the treated rabbits were observed. Plasma urea, uric acid, creatinine and blood glucose levels increased significantly (p < 0.05) in the treated rabbits. Keywords : Histopathological, Phyllanthus niruri, Saponin, Toxicological. GJMR-B Classification : NLMC Code: WX 207, QV 290 HistopathologicalandToxicologicaleffectsofcrudesaponinextractfromPhyllanthusniruri,LSyn.P.franternus.WebsteronOrgansinanimalstudies Strictly as per the compliance and regulations of: © 2012 Ajibade V. A., Famurewa, O. This is a research/review paper, distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction inany medium, provided the original work is properly cited. -

10 Medicinal Plants from Pakistan
10 MEDICINAL PLANTS OF PAKISTAN A LITERATURE STUDY BY, MOHAMMAD AWAIS INSTITUTE OF PHARMACY THE FACULTY OF MATHEMATICS AND NATURAL SCIENCES THE UNIVERSITY OF OSLO (NORWAY) DESEMBER, 2008 2 10 MEDICINAL PLANTS OF PAKISTAN A LITERATURE STUDY THESIS IN PHARMACOGNOSY BY, MOHAMMAD AWAIS INSTITUTE OF PHARMACY THE FACULTY OF MATHEMATICS AND NATURAL SCIENCES THE UNIVERSITY OF OSLO INSTRUCTOR Professor Ph.D. Berit Smestad Paulsen Department of Pharmaceutical chemistry, Institute of Pharmacy, The University of Oslo (Norway), December 2008. 3 CONTENTS PREFACE ................................................................................................................................................ 17 10 SELECTED MEDICINAL PLANTS OF PAKISTAN ................................................................................... 18 INTRODUCTION ..................................................................................................................................... 19 ISLAMIC REPUBLIC OF PAKISTAN ........................................................................................................... 19 MEDICINAL PLANTS OF PAKISTAN ......................................................................................................... 20 BACKGROUND LITERATURE ................................................................................................................... 20 STRUCTURE OF THESIS .......................................................................................................................... 21 LITERATURE REFERENCES -

Medicinal Herbs Quick Reference Guide Revision 2*
Medicinal Herbs Quick Reference Guide * Revision 2 Julieta Criollo DNM, CHT Doctor of Natural Medicine Clinical Herbal Therapist Wellness Trading Post [email protected] www.wellnesstradingpost.com 604-760-6425 Copyright © 2004–2011 Julieta Criollo All rights reserved. Note to the reader This booklet is intended for educational purposes only. The information contained in it has been compiled from published books and material on plant medicine. Although the information has been reviewed for correctness, the publisher/author does not assume any legal responsibility and/or liability for any errors or omissions. Furthermore, herbal/plant medicine standards (plant identification, medicinal properties, preparations, dosage, safety precautions and contraindications, pharmacology, and therapeutic usage) are continuously evolving and changing as new research and clinical studies are being published and expanding our knowledge. Hence, readers are encouraged and advised to check the most current information available on plant medicine standards and safety. T his information is not intended to be a substitute for professional medical advice. Always seek the advice of a medical doctor or qualified health practitioner prior to starting any new treatment, or with any questions you may have regarding a medical condition. The publisher/author does not assume any legal responsibility and/or liability for the use of the information contained in this booklet. * Revision 1 updates: addition of color bars to the Herb Groups and the various Herb -

Studies on Betalain Phytochemistry by Means of Ion-Pair Countercurrent Chromatography
STUDIES ON BETALAIN PHYTOCHEMISTRY BY MEANS OF ION-PAIR COUNTERCURRENT CHROMATOGRAPHY Von der Fakultät für Lebenswissenschaften der Technischen Universität Carolo-Wilhelmina zu Braunschweig zur Erlangung des Grades einer Doktorin der Naturwissenschaften (Dr. rer. nat.) genehmigte D i s s e r t a t i o n von Thu Tran Thi Minh aus Vietnam 1. Referent: Prof. Dr. Peter Winterhalter 2. Referent: apl. Prof. Dr. Ulrich Engelhardt eingereicht am: 28.02.2018 mündliche Prüfung (Disputation) am: 28.05.2018 Druckjahr 2018 Vorveröffentlichungen der Dissertation Teilergebnisse aus dieser Arbeit wurden mit Genehmigung der Fakultät für Lebenswissenschaften, vertreten durch den Mentor der Arbeit, in folgenden Beiträgen vorab veröffentlicht: Tagungsbeiträge T. Tran, G. Jerz, T.E. Moussa-Ayoub, S.K.EI-Samahy, S. Rohn und P. Winterhalter: Metabolite screening and fractionation of betalains and flavonoids from Opuntia stricta var. dillenii by means of High Performance Countercurrent chromatography (HPCCC) and sequential off-line injection to ESI-MS/MS. (Poster) 44. Deutscher Lebensmittelchemikertag, Karlsruhe (2015). Thu Minh Thi Tran, Tamer E. Moussa-Ayoub, Salah K. El-Samahy, Sascha Rohn, Peter Winterhalter und Gerold Jerz: Metabolite profile of betalains and flavonoids from Opuntia stricta var. dilleni by HPCCC and offline ESI-MS/MS. (Poster) 9. Countercurrent Chromatography Conference, Chicago (2016). Thu Tran Thi Minh, Binh Nguyen, Peter Winterhalter und Gerold Jerz: Recovery of the betacyanin celosianin II and flavonoid glycosides from Atriplex hortensis var. rubra by HPCCC and off-line ESI-MS/MS monitoring. (Poster) 9. Countercurrent Chromatography Conference, Chicago (2016). ACKNOWLEDGEMENT This PhD would not be done without the supports of my mentor, my supervisor and my family. -

Nemomex Nematicide SAFETY DATA SHEET
NemOmex Nematicide SAFETY DATA SHEET Section 1: Identification of the substance/mixture and of the company/undertaking 1.1.1.2. 1.1. Product name : NemOmex Nematicide Identification : EPA Reg. No. 82572-1-86868 CAS number : 68990-67-0 EINECS number : 273-620-4 USA (FDA) : E999 21 CFR 172.510. FEMA GRAS NUMBER 2973. 1.3.1.4. 1.2. Relevant identified uses of substance or mixture and uses advised against. Wetting agent. 1.5.1.6. 1.3. Details of supplier for the safety data sheet. Omex Agrifluids, Inc. 1675 Dockery Avenue Selma, CA 93662 1.7.1.8. 1.4. Emergency telephone number. 1-800-424-9300 Section 2: Hazards identification 2.1. Product definition: Quillaja saponaria wood extract liquid. 2.2. Classification of the substance or mixture. According to Directive criteria, 1999/45/EC and 67/548/EEC the following amendments thereof: Xi : Irritant. R Phases : R36 Irritating to eyes. S Phrases : S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Regulation criteria 1272/2008 (CLP/GHS): : warning. Hazard Statements : H320. Causes eye irritation. Precautionary Statements: P264 : Wash with water the parts of your body that had contact with the product. P280 : Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 : IF IN EYES. Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do so, continue rinsing. P314 : Get medical advice/attention if you feel unwell. P362 : Take off contaminated clothing and wash before reuse. Page 1 of 9 NemOmex Nematicide P501 : Dispose of contents/container in accordance with applicable regulations. -

Contributions to the Solution of Phylogenetic Problem in Fabales
Research Article Bartın University International Journal of Natural and Applied Sciences Araştırma Makalesi JONAS, 2(2): 195-206 e-ISSN: 2667-5048 31 Aralık/December, 2019 CONTRIBUTIONS TO THE SOLUTION OF PHYLOGENETIC PROBLEM IN FABALES Deniz Aygören Uluer1*, Rahma Alshamrani 2 1 Ahi Evran University, Cicekdagi Vocational College, Department of Plant and Animal Production, 40700 Cicekdagi, KIRŞEHIR 2 King Abdulaziz University, Department of Biological Sciences, 21589, JEDDAH Abstract Fabales is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae), Polygalaceae, Surianaceae and Quillajaceae. The monophyly of the order is supported strongly by several studies, although interfamilial relationships are still poorly resolved and vary between studies; a situation common in higher level phylogenetic studies of ancient, rapid radiations. In this study, we carried out simulation analyses with previously published matK and rbcL regions. The results of our simulation analyses have shown that Fabales phylogeny can be solved and the 5,000 bp fast-evolving data type may be sufficient to resolve the Fabales phylogeny question. In our simulation analyses, while support increased as the sequence length did (up until a certain point), resolution showed mixed results. Interestingly, the accuracy of the phylogenetic trees did not improve with the increase in sequence length. Therefore, this study sounds a note of caution, with respect to interpreting the results of the “more data” approach, because the results have shown that large datasets can easily support an arbitrary root of Fabales. Keywords: Data type, Fabales, phylogeny, sequence length, simulation. 1. Introduction Fabales Bromhead is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae) Juss., Polygalaceae Hoffmanns. -

FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast
FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast Beverages Entrée - Bowls/Mac/Flatbread Pizza Kids Dressings Pastries & Sweets Salads Sandwiches Sides Smoothies Souffles Soups Spreads Grab N Go Catering ©2021 Panera Bread. All Rights Reserved. Bakery - Cafe Nutrition, Ingredient, and Allergen Information Effective 1/13/2021 Version 2 Bagels Asiago Cheese Bagel g) m ( l mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat (g) m i i i ASIAGO CHEESE BAGEL (ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, s ra u i ar REDUCED IRON, THIAMINE MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, ASIAGO CHEESE u (g) ole lor lor rbs (g) g d t a a h a (PASTEURIZED MILK, CHEESE CULTURE, SALT, MICROBIAL AND ANIMAL ENZYMES, POWDERED nts Servin C C Fa Sat Tran C So C Fiber (g) Su Prote CELLULOSE [TO PREVENT CAKING]), BROWN SUGAR, SALT, DOUGH IMPROVER (MALTED e i WHEAT FLOUR, ENRICHED WHEAT FLOUR [NIACIN, REDUCED IRON, THIAMINE MONONITRATE, d RIBOFLAVIN, FOLIC ACID], INACTIVATED YEAST, ACEROLA EXTRACT, FUNGAL ENZYMES), YEAST e (YEAST, SORBITAN MONOSTEARATE, ASCORBIC ACID)), ASIAGO CHEESE (PASTEURIZED MILK, 1 Bagel 320 50 5 3 0 15 530 55 2 4 14 CHEESE CULTURES, SALT, MICROBIAL & ANIMAL ENZYMES, POWDERED CELLULOSE [TO PREVENT CAKING]). Ingr CONTAINS MILK, WHEAT. Allergens * Blueberry Bagel g) m ) ( l g mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat ( m i i i ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, REDUCED IRON, THIAMINE n s ra u i i ar MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, BLUEBERRY FLAVORED