Telomere Elongation in Regenerating Tissues of the Grey Starfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity and Phylogeography of Southern Ocean Sea Stars (Asteroidea)
Diversity and phylogeography of Southern Ocean sea stars (Asteroidea) Thesis submitted by Camille MOREAU in fulfilment of the requirements of the PhD Degree in science (ULB - “Docteur en Science”) and in life science (UBFC – “Docteur en Science de la vie”) Academic year 2018-2019 Supervisors: Professor Bruno Danis (Université Libre de Bruxelles) Laboratoire de Biologie Marine And Dr. Thomas Saucède (Université Bourgogne Franche-Comté) Biogéosciences 1 Diversity and phylogeography of Southern Ocean sea stars (Asteroidea) Camille MOREAU Thesis committee: Mr. Mardulyn Patrick Professeur, ULB Président Mr. Van De Putte Anton Professeur Associé, IRSNB Rapporteur Mr. Poulin Elie Professeur, Université du Chili Rapporteur Mr. Rigaud Thierry Directeur de Recherche, UBFC Examinateur Mr. Saucède Thomas Maître de Conférences, UBFC Directeur de thèse Mr. Danis Bruno Professeur, ULB Co-directeur de thèse 2 Avant-propos Ce doctorat s’inscrit dans le cadre d’une cotutelle entre les universités de Dijon et Bruxelles et m’aura ainsi permis d’élargir mon réseau au sein de la communauté scientifique tout en étendant mes horizons scientifiques. C’est tout d’abord grâce au programme vERSO (Ecosystem Responses to global change : a multiscale approach in the Southern Ocean) que ce travail a été possible, mais aussi grâce aux collaborations construites avant et pendant ce travail. Cette thèse a aussi été l’occasion de continuer à aller travailler sur le terrain des hautes latitudes à plusieurs reprises pour collecter les échantillons et rencontrer de nouveaux collègues. Par le biais de ces trois missions de recherches et des nombreuses conférences auxquelles j’ai activement participé à travers le monde, j’ai beaucoup appris, tant scientifiquement qu’humainement. -

Systematics, Phylogeny and Historical Biogeography of the Pentagonaster Clade (Asteroidea: Valvatida: Goniasteridae)
CSIRO PUBLISHING www.publish.csiro.au/journals/is Invertebrate Systematics, 2007, 21,311—339 Systematics, phylogeny and historical biogeography of the Pentagonaster clade (Asteroidea: Valvatida: Goniasteridae) Christopher Mah Department of Invertebrate Zoology, National Museum of Natural History, MRC-1 63, PO Box 3701 2 Smithsonian Institution, Washington, DC 20560, USA. Email: [email protected] Abstract. Morphology-based phylogenetic hypotheses developed for living and fossil goniasterid asteroids have pro- vided several unique opportunities to study bathymetric and biogeographic shifts for an ecologically important group of prominent, megafaunal invertebrates. A cladistic analysis of 18 ingroup taxa employing 65 morphological characters resulted in a single most parsimonious tree. The tree supports assignment of the Atlantic Tosia parva (Perrier, 1881) and the Pacific Tosia queenslandensis Livingstone, 1932 to new, separate genera. The phylogenetic tree supports offshore to onshore bathymetric shifts between basal and derived taxa. The phylogeny is also consistent with historical events sur- rounding the separation of Antarctica from Australia and South Africa. Buterminaster Blake & Zinsmeister, 1988 from the Eocene La Meseta Formation, Antarctic Peninsula, was included in the phylogenetic analysis and is now supported as the only fossil species in the genus Pentagonaster Gray, 1840. Pentagonaster stibarus H. L. Clark, 1914 is separated from syn- onymy with P. dubeni Gray, 1847 and resurrected as a valid species. The new genus, Akelbaster, gen. nov, shows unusual new structures that resemble cribiform organs, although their function has not been determined. One specific ingroup lineage, including Tosia and Pentagonaster, attains a much larger adult size than those of its sister-taxa, suggesting that Cope's rule may apply to asteroids within this clade. -

The Position of the Ophiuroidea Within the Phylum Echinodermata
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 2005 The Position of the Ophiuroidea within the Phylum Echinodermata Mary C. Harmon University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Harmon, Mary C., "The Position of the Ophiuroidea within the Phylum Echinodermata" (2005). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/2916 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. The Position of the Ophiuroidea within the Phylum Echinodermata by Mary C. Harmon A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Biology College of Arts and Sciences University of South Florida Major Professor: Brian T. Livingston, Ph.D. James R. Garey, Ph.D. Jessica L. Moore, Ph.D. Date of Approval: November 18, 2005 Keywords: molecular phylogeny, evolution, echinoderm classes, ribosomal DNA, ophiuroid © Copyright 2005, Mary C. Harmon Dedication For my parents, who have instilled in me the desire to succeed, and given me the tools necessary to do so. For Holly and my friends and family. Thank you for believing in me and for providing enjoyable breaks from my scholarly chores when I needed them. And when I didn’t. For Ailey Marie. 26 December 1998 - 07 October 2005. She taught me many important things…none of which were related to echinoderms. -

Contributions to the Classification of the Sea-Stars of Japan. : II
Contributions to the Classification of the Sea-stars of Japan. : II. Forcipulata, with the Note on the Relationships Title between the Skeletal Structure and Respiratory Organs of the Sea-stars (With 11 Plates and 115 textfigures) Author(s) HAYASHI, Ryoji Citation 北海道帝國大學理學部紀要, 8(3), 133-281 Issue Date 1943-03 Doc URL http://hdl.handle.net/2115/27045 Type bulletin (article) File Information 8(3)_P133-281.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP , \ Contributio~s to the Classification of the Sea"stars ,of Japan. n. Forclpulata, with the Note on the Rela.. tionships between the Skeletal Structure and Respiratory Organs of the Sea"starsU Ryoji Hayashi Research Institute for Natural Resources (With 11 plates and 115 te::etjigures) It is the second report o{ the writer's investigation on the sea stars of Japan, undertaken under the guidance of Prof. Tohru Uchida and contains the following 41 forms belonging to the three families, Brisingidae, Zoroasteridae and Asteriidae. These families are all included in the .order Forcipulata. From Japanese waters 18 species of Forcipulata have previously been reported. 'l'hey all belong to the family Asteriidae, except Sladen's two species of Brisingidae. , The species newly recorde~ are marked with asterisk. Family Brisingidae * Odinia pacifica forma sagamiana n. forma * Odinia aust't'ni forma japonioo n. forma * Parabrisinga, pellucida n. sp. ' Brisingellaarmillata (SLADEN) * Freyellaster Fecundus forma ochotJensis n. forma * Freyellaster mtermedius n. sp. Freyella pennata SLADEN Family Zoroasteri~e * Zoroaster orientalis n. sp. * Zoroasterorientalis n. sp. forma gracilis n. forma * Zoroaster ophia.ctis FISHER * Zoroaster micropoTus FISHER * Cnemidaster 'wyvillii .SLADEN" 1) : Contributions from the Akkeshi Marine Biologi~al Station, No. -

Environmental Surveys of Potential Borrow Areas on the Central East
OCS Study MMS 2004-037 FINAL REPORT ENVIRONMENTAL SURVEYS OF POTENTIAL BORROW AREAS ON THE CENTRAL EAST FLORIDA SHELF AND THE ENVIRONMENTAL IMPLICATIONS OF SAND REMOVAL FOR COASTAL AND BEACH RESTORATION Contract Number 1435-01-00-CT-31044 Study Area NASA IMAGE Prepared For: Prepared By: U.S. Department of the Interior Continental Shelf Associates, Inc. Minerals Management Service 759 Parkway Street Leasing Division Jupiter, Florida 33477 Marine Minerals Branch In Cooperation With: Applied Coastal Research and Engineering, Inc. Barry A. Vittor & Associates, Inc. Florida Geological Survey DISCLAIMER This manuscript has been reviewed by the Minerals Management Service and approved for publication. The opinions, findings, conclusions, and recommendations expressed in this report are those of the authors, and do not necessarily reflect the views or policies of the MMS. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This report has been technically reviewed according to contractual specifications; however, it is exempt from further review by the MMS Technical Publication Unit. SUGGESTED CITATION Hammer, R.M., M.R. Byrnes, D.B. Snyder, T.D. Thibaut, J.L. Baker, S.W. Kelley, J.M. Côté, L.M. Lagera, Jr., S.T. Viada, B.A. Vittor, J.S. Ramsey, and J.D. Wood, 2005. Environmental Surveys of Potential Borrow Areas on the Central East Florida Shelf and the Environmental Implications of Sand Removal for Coastal and Beach Restoration. Prepared by Continental Shelf Associates, Inc. in cooperation with Applied Coastal Research and Engineering, Inc., Barry A. Vittor & Associates, Inc., and the Florida Geological Survey for the U.S. -
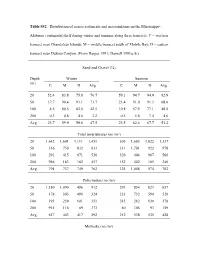
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg. -

In the Azores Archipelago (NE Atlantic Ocean)
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 First record of the Mediterranean asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) in the Azores Archipelago (NE Atlantic Ocean) PATRÍCIA MADEIRA, A.M. DE FRIAS MARTINS & S.P. ÁVILA Madeira, P., A.M. de Frias Martins & S.P. Ávila 2017. First record of the Mediterranean asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) in the Azores Archipelago (NE Atlantic Ocean). Arquipelago. Life and Marine Sciences 35: 11-18. The first occurrence of the Mediterranean fissiparous asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) is reported from the Azores based upon dredged material off the south coast of São Miguel Island at 135 m depth. This record represents a considerable expansion of the species’ geographic range, otherwise reported with certainty only from the Mediterranean Sea. S. richardi is capable of producing long-lived planktotrophic larvae with high dispersal potential to reach remote areas such as the Azores. Alternatively, this species is also capable of reproducing asexually through fission, which could insure the maintenance of viable numbers in a stranded population. The presence of S. richardi in Azorean waters and its rarity in an otherwise thoroughly investigated area does not necessarily imply a recent arrival nor a human-mediated introduction, as the depths in consideration (80-700 m) are also the least studied in the archipelago. Key words: Asteroidea, Forcipulatida, fissiparous, Azores. Patrícia Madeira1,2([email protected]), A.M. de Frias Martins2 & S.P. Ávila1,2. 1CIBIO – Research Centre in Biodiversity and Genetic Resources, InBIO/Azores Associate Laboratory, Faculty of Sciences & Technology, Campus of Ponta Delgada, Azores, Portugal. -

In the Azores Archipelago (NE Atlantic Ocean)
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 First record of the Mediterranean asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) in the Azores Archipelago (NE Atlantic Ocean) PATRÍCIA MADEIRA, A.M. DE FRIAS MARTINS & S.P. ÁVILA Madeira, P., A.M. de Frias Martins & S.P. Ávila 2017. First record of the Mediterranean asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) in the Azores Archipelago (NE Atlantic Ocean). Arquipelago. Life and Marine Sciences 35: 11-18. The first occurrence of the Mediterranean fissiparous asteroid Sclerasterias richardi (Perrier in Milne-Edwards 1882) is reported from the Azores based upon dredged material off the south coast of São Miguel Island at 135 m depth. This record represents a considerable expansion of the species’ geographic range, otherwise reported with certainty only from the Mediterranean Sea. S. richardi is capable of producing long-lived planktotrophic larvae with high dispersal potential to reach remote areas such as the Azores. Alternatively, this species is also capable of reproducing asexually through fission, which could insure the maintenance of viable numbers in a stranded population. The presence of S. richardi in Azorean waters and its rarity in an otherwise thoroughly investigated area does not necessarily imply a recent arrival nor a human-mediated introduction, as the depths in consideration (80-700 m) are also the least studied in the archipelago. Key words: Asteroidea, Forcipulatida, fissiparous, Azores. Patrícia Madeira1,2([email protected]), A.M. de Frias Martins2 & S.P. Ávila1,2. 1CIBIO – Research Centre in Biodiversity and Genetic Resources, InBIO/Azores Associate Laboratory, Faculty of Sciences & Technology, Campus of Ponta Delgada, Azores, Portugal. -

Niche Modeling Remarks of Luidia Senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) After 30 Years of Its First Capture in the Northeastern Brazilian Coast
Latin American Journal of Aquatic Research, 48Niche(3): 497 modeling-505, 2020 remarks of Luidia senegalensis 497 DOI: 10.3856/vol48-issue3-fulltext-2130 Short communication Niche modeling remarks of Luidia senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) after 30 years of its first capture in the northeastern Brazilian coast Carolina T. Puppin-Gonçalves1, Matheus Arthur L. Rocha1, Carlos E.R.D. Alencar1,2 1 1,3 1 Sávio A.S.N. Moraes , Paulo V.N. Araújo & Fúlvio A.M. Freire 1Laboratório de Ecologia e Evolução de Crustáceos (LABEEC), Departamento de Biologia Ecologia e Zoologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte (UFRN) Campus Universitário Lagoa Nova, Natal, Brasil 2Departamento de Ciências Biológicas, Universidade Regional do Cariri (URCA) Campus Pimenta Crato, Brasil 3Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Norte (IFRN) Campus Macau, Macau, Brasil Corresponding author: Carolina T. Puppin-Gonçalves ([email protected]) ABSTRACT. After more than 30 years of species' first capture on the Brazilian northeast coast, we report the second occurrence of the starfish Luidia senegalensis with niche modeling remarks on its distribution. Bottom trawl net collected specimens with artisanal fishery boat in Rio Grande do Norte State, northeast Brazil. It was noted the existence of a large number of regions, with high suitability for the occurrence of this species, in South America taking into account the ecological niche modeling, when compared to North and Central American continents. Benthic salinity range, calcite, and benthic minimum temperature were the most relevant for modeling. The northeastern, eastern, and southeastern Brazil ecoregions showed the most considerable amount of areas with high suitability for L. -

FEP Volume II Calico Scallop
To date there has been no attempt at a comprehensive stock assessment for wahoo. Therefore, the status of the stocks is unknown at this time. Proxy MSY estimates were provided by the NMFS SEFSC and were used to specify the status determination criteria in the Dolphin Wahoo FMP. 4.1.9 Calico Scallops Description and Distribution Calico scallops, Argopecten gibbus (Linnaeus 1758), are part of the bivalve mollusc family Pectinidae that contains all commercial species of scallops (Waller 1991). They are unified by series of minute denticles formed in the notch of the right valve, most visible in early juvenile stages. Waller (2006) indicates there are four major groupings or subfamilies, three of which are monophyletic (Camptonectinae, Palliolinae and Pectininae) and one of which is paraphyletic (Chlamydinae). At least six species in the subfamily Pectininae are commercially exploited: Aequipecten operularis (queen scallop), Argopecten irradians (bay scallops) and A. gibbus in the North Atlantic, Aequipecten tehuelchus (Tehuleche scallop) in the South Atlantic, and Argopecten purpuratus (Chilean scallop) and A. ventricosus (Catarina scallop) in the eastern Pacific. Identification of calico scallops can be made from shell color and morphology. The upper (left) valve has red or maroon calico markings over a white or yellow base; the lower (right valve) is more lightly pigmented. The calico markings on the shell distinguish this scallop from the solid gray or brown upper valve of the bay scallop, which resembles the calico scallop in size. Calico scallop shell morphology varies with locality (Krause et al. 1994), but generally the species reaches 40 to 60 mm (1.6-2.4 in) in shell height (a straight line measurement of the greatest distance between the umbo and the ventral margin), with a maximum size reported to be about 80 mm (3.2 in) in shell diameter (a straight line measurement of the greatest distance between the anterior and posterior margin) (Roe et al. -

4-Araujo 64.Indd
VIE ET MILIEU - LIFE AND ENVIRONMENT, 2014, 64: 35-46 A trophic ANalYsis of target species of macrobeNthos IN A SUBTROPICAL coastal COMMUNITY: A taXA relatioNSHIP ESSAY M. E. DE ARAÚJO 1, M. J. LUNARDON-BRANCO 2, J. R. VERANI 3, J. O. BRANCO 2, J. P. BARREIROS 4* & M. L. CHRISTOFFERSEN 5 1 Universidade Federal de Pernambuco, CTG, Departamento de Oceanografia, CEP 50.740-550, Recife, Pernambuco, Brazil 2 Centro de Ciências Tecnológicas da Terra e do Mar, Universidade Vale do Itajaí, CP 360, CEP 88.302-202, Itajaí, Santa Catarina, Brazil 3 Universidade Federal de São Carlos. Cx. Postal 676, CEP 13565-905 São Carlos, SP, Brazil 4 Azorean Biodiversity Group (CITA-A) and Platform for Enhancing Ecological Research & Sustainability (PEERS), Universidade dos Açores, Dep. Ciências Agrárias, 9700-042 Angra do Heroísmo, Portugal 5 Universidade Federal da Paraíba, Departamento de Sistemática e Ecologia, CEP 58.059-900, João Pessoa, Paraíba, Brazil * Corresponding author: [email protected] CLADOGRAM ABSTRACT. – Studies on the feeding habits of aquatic organisms are a requirement for the COMMON FOOD ITEMS TROPHIC RELATIONS management and sustainable use of marine ecosystems. The aim of the present research was to analyze the habits and trophic similarities of decapods, starfish and fish in order to propose trophic relationships between taxa, using Hennigian methods of phylogenetic systematics. This new grouping hypothesis, based on shared and exclusive food items and food types, corresponds to the broad taxonomic groups used in the analysis. Our results indicate that algae, Mollusca, Polychaeta, Crustacea, Echinodermata and Actinopterygii are the most exploited common resources among the species studied. -

Non-Genetic Inheritance and Changing Environments
NON-GENETIC INHERITANCE Mini-review • DOI: 10.2478/ngi-2013-0005 • NGI • 2012 • 38-50 Non-genetic inheritance and changing environments Abstract Santiago Salinas1*, Marc Mangel1,3, Climate change continues to impact species worldwide. Understanding Simon C. Brown2, Stephan B. Munch4 and predicting how populations will respond is of clear importance. Here, we review a mechanism by which populations may respond rapidly to these changes: Trans-Generational Plasticity (TGP). TGP exists when the 1Center for Stock Assessment Research, 3Dept. of Biology, University of Bergen, environment experienced by the parents affects the shape of the reaction University of California Santa Cruz, Bergen, 5020, Norway norm in their offspring; that is, the parental and offspring environments Santa Cruz, CA 95060, USA interact to determine the offspring phenotype. We survey 80 empirical 4 2Dept. of Ecology and Evolutionary Biology, Fisheries Ecology Division, studies from 63 species (32 orders, 9 phyla) that demonstrate TGP. Overall, University of California Santa Cruz, Southwest Fisheries Science Center, TGP is taxonomically widespread and present in response to environmental Santa Cruz, CA, 95060, USA Santa Cruz, CA, 95060, USA drivers likely to be impacted by climate change. Although many examples now exist, we also identify areas of research that could greatly improve our understanding of TGP. We conclude that TGP is sufficiently established both theoretically and empirically to merit study as a potential coping tactic against rapid environmental changes.