4-Araujo 64.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Webs of Mediterranean Coastal Wetlands
FOOD WEBS OF MEDITERRANEAN COASTAL WETLANDS Jordi COMPTE CIURANA ISBN: 978-84-693-8422-0 Dipòsit legal: GI-1204-2010 http://www.tdx.cat/TDX-1004110-123344 ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tesisenxarxa.net) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tesisenred.net) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR. No se autoriza la presentación de su contenido en una ventana o marco ajeno a TDR (framing). Esta reserva de derechos afecta tanto al resumen de presentación de la tesis como a sus contenidos. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

The Position of the Ophiuroidea Within the Phylum Echinodermata
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 2005 The Position of the Ophiuroidea within the Phylum Echinodermata Mary C. Harmon University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Harmon, Mary C., "The Position of the Ophiuroidea within the Phylum Echinodermata" (2005). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/2916 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. The Position of the Ophiuroidea within the Phylum Echinodermata by Mary C. Harmon A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Biology College of Arts and Sciences University of South Florida Major Professor: Brian T. Livingston, Ph.D. James R. Garey, Ph.D. Jessica L. Moore, Ph.D. Date of Approval: November 18, 2005 Keywords: molecular phylogeny, evolution, echinoderm classes, ribosomal DNA, ophiuroid © Copyright 2005, Mary C. Harmon Dedication For my parents, who have instilled in me the desire to succeed, and given me the tools necessary to do so. For Holly and my friends and family. Thank you for believing in me and for providing enjoyable breaks from my scholarly chores when I needed them. And when I didn’t. For Ailey Marie. 26 December 1998 - 07 October 2005. She taught me many important things…none of which were related to echinoderms. -

Worms, Germs, and Other Symbionts from the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor
h ' '' f MASGC-B-78-001 c. 3 A MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor NATIONAL SEA GRANT DEPOSITORY \ PELL LIBRARY BUILDING URI NA8RAGANSETT BAY CAMPUS % NARRAGANSETT. Rl 02882 Robin M. Overstreet r ii MISSISSIPPI—ALABAMA SEA GRANT CONSORTIUM MASGP—78—021 MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico by Robin M. Overstreet Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 This study was conducted in cooperation with the U.S. Department of Commerce, NOAA, Office of Sea Grant, under Grant No. 04-7-158-44017 and National Marine Fisheries Service, under PL 88-309, Project No. 2-262-R. TheMississippi-AlabamaSea Grant Consortium furnish ed all of the publication costs. The U.S. Government is authorized to produceand distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon. Copyright© 1978by Mississippi-Alabama Sea Gram Consortium and R.M. Overstrect All rights reserved. No pari of this book may be reproduced in any manner without permission from the author. Primed by Blossman Printing, Inc.. Ocean Springs, Mississippi CONTENTS PREFACE 1 INTRODUCTION TO SYMBIOSIS 2 INVERTEBRATES AS HOSTS 5 THE AMERICAN OYSTER 5 Public Health Aspects 6 Dcrmo 7 Other Symbionts and Diseases 8 Shell-Burrowing Symbionts II Fouling Organisms and Predators 13 THE BLUE CRAB 15 Protozoans and Microbes 15 Mclazoans and their I lypeiparasites 18 Misiellaneous Microbes and Protozoans 25 PENAEID -

Environmental Surveys of Potential Borrow Areas on the Central East
OCS Study MMS 2004-037 FINAL REPORT ENVIRONMENTAL SURVEYS OF POTENTIAL BORROW AREAS ON THE CENTRAL EAST FLORIDA SHELF AND THE ENVIRONMENTAL IMPLICATIONS OF SAND REMOVAL FOR COASTAL AND BEACH RESTORATION Contract Number 1435-01-00-CT-31044 Study Area NASA IMAGE Prepared For: Prepared By: U.S. Department of the Interior Continental Shelf Associates, Inc. Minerals Management Service 759 Parkway Street Leasing Division Jupiter, Florida 33477 Marine Minerals Branch In Cooperation With: Applied Coastal Research and Engineering, Inc. Barry A. Vittor & Associates, Inc. Florida Geological Survey DISCLAIMER This manuscript has been reviewed by the Minerals Management Service and approved for publication. The opinions, findings, conclusions, and recommendations expressed in this report are those of the authors, and do not necessarily reflect the views or policies of the MMS. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This report has been technically reviewed according to contractual specifications; however, it is exempt from further review by the MMS Technical Publication Unit. SUGGESTED CITATION Hammer, R.M., M.R. Byrnes, D.B. Snyder, T.D. Thibaut, J.L. Baker, S.W. Kelley, J.M. Côté, L.M. Lagera, Jr., S.T. Viada, B.A. Vittor, J.S. Ramsey, and J.D. Wood, 2005. Environmental Surveys of Potential Borrow Areas on the Central East Florida Shelf and the Environmental Implications of Sand Removal for Coastal and Beach Restoration. Prepared by Continental Shelf Associates, Inc. in cooperation with Applied Coastal Research and Engineering, Inc., Barry A. Vittor & Associates, Inc., and the Florida Geological Survey for the U.S. -

Copyright© 2018 Mediterranean Marine Science
Mediterranean Marine Science Vol. 19, 2018 Hazeus ingressus sp. nov. a new goby species (Perciformes: Gobiidae) and a new invasion in the Mediterranean Sea ENGIN SEMIH Izmir Katip Celebi University, Faculty of Fisheries, Havaalanı Sosesi Cd. No:33/2, 35620 Cigli/Izmir/Turkey LARSON HELEN Museum and Art Gallery of the Northern Territory, P.O. Box 4646, Darwin, Northern Territory 0801, Australia; Museum of Tropical Queensland, 102 Flinders Street, Townsville, Queensland 4810, Australia IRMAK ERHAN Izmir Katip Celebi University, Faculty of Fisheries, Havaalanı Sosesi Cd. No:33/2, 35620 Cigli/Izmir/Turkey http://dx.doi.org/10.12681/mms.14336 Copyright © 2018 Mediterranean Marine Science To cite this article: ENGIN, S., LARSON, H., & IRMAK, E. (2018). Hazeus ingressus sp. nov. a new goby species (Perciformes: Gobiidae) and a new invasion in the Mediterranean Sea. Mediterranean Marine Science, 19(2), 316-325. doi:http://dx.doi.org/10.12681/mms.14336 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 27/06/2019 17:49:13 | Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available online at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.14336 Hazeus ingressus sp. nov. a new goby species (Perciformes: Gobiidae) and a new invasion in the Mediterranean Sea SEMIH ENGIN1, HELEN LARSON2 and ERHAN IRMAK1 1İzmir Katip Celebi University, Faculty of Fisheries, Havaalanı Sosesi Cd. No:33/2, 35620 Cigli, Izmir, Turkey 2Museum and Art Gallery of the Northern Territory, P.O. Box 4646, Darwin, Northern Territory 0801, Australia; Museum of Tropical Queensland, 102 Flinders Street, Townsville, Queensland 4810, Australia Corresponding author: [email protected] Handling Editor: Murat Bilecenoglu Received: 14 August 2017; Accepted: 6 May 2018; Published on line: 5 July 2018 Abstract A new species of gobiid, Hazeus ingressus sp. -
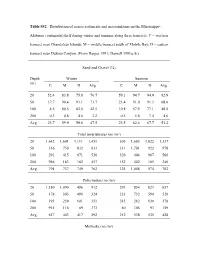
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg. -

Niche Modeling Remarks of Luidia Senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) After 30 Years of Its First Capture in the Northeastern Brazilian Coast
Latin American Journal of Aquatic Research, 48Niche(3): 497 modeling-505, 2020 remarks of Luidia senegalensis 497 DOI: 10.3856/vol48-issue3-fulltext-2130 Short communication Niche modeling remarks of Luidia senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) after 30 years of its first capture in the northeastern Brazilian coast Carolina T. Puppin-Gonçalves1, Matheus Arthur L. Rocha1, Carlos E.R.D. Alencar1,2 1 1,3 1 Sávio A.S.N. Moraes , Paulo V.N. Araújo & Fúlvio A.M. Freire 1Laboratório de Ecologia e Evolução de Crustáceos (LABEEC), Departamento de Biologia Ecologia e Zoologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte (UFRN) Campus Universitário Lagoa Nova, Natal, Brasil 2Departamento de Ciências Biológicas, Universidade Regional do Cariri (URCA) Campus Pimenta Crato, Brasil 3Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Norte (IFRN) Campus Macau, Macau, Brasil Corresponding author: Carolina T. Puppin-Gonçalves ([email protected]) ABSTRACT. After more than 30 years of species' first capture on the Brazilian northeast coast, we report the second occurrence of the starfish Luidia senegalensis with niche modeling remarks on its distribution. Bottom trawl net collected specimens with artisanal fishery boat in Rio Grande do Norte State, northeast Brazil. It was noted the existence of a large number of regions, with high suitability for the occurrence of this species, in South America taking into account the ecological niche modeling, when compared to North and Central American continents. Benthic salinity range, calcite, and benthic minimum temperature were the most relevant for modeling. The northeastern, eastern, and southeastern Brazil ecoregions showed the most considerable amount of areas with high suitability for L. -

FEP Volume II Calico Scallop
To date there has been no attempt at a comprehensive stock assessment for wahoo. Therefore, the status of the stocks is unknown at this time. Proxy MSY estimates were provided by the NMFS SEFSC and were used to specify the status determination criteria in the Dolphin Wahoo FMP. 4.1.9 Calico Scallops Description and Distribution Calico scallops, Argopecten gibbus (Linnaeus 1758), are part of the bivalve mollusc family Pectinidae that contains all commercial species of scallops (Waller 1991). They are unified by series of minute denticles formed in the notch of the right valve, most visible in early juvenile stages. Waller (2006) indicates there are four major groupings or subfamilies, three of which are monophyletic (Camptonectinae, Palliolinae and Pectininae) and one of which is paraphyletic (Chlamydinae). At least six species in the subfamily Pectininae are commercially exploited: Aequipecten operularis (queen scallop), Argopecten irradians (bay scallops) and A. gibbus in the North Atlantic, Aequipecten tehuelchus (Tehuleche scallop) in the South Atlantic, and Argopecten purpuratus (Chilean scallop) and A. ventricosus (Catarina scallop) in the eastern Pacific. Identification of calico scallops can be made from shell color and morphology. The upper (left) valve has red or maroon calico markings over a white or yellow base; the lower (right valve) is more lightly pigmented. The calico markings on the shell distinguish this scallop from the solid gray or brown upper valve of the bay scallop, which resembles the calico scallop in size. Calico scallop shell morphology varies with locality (Krause et al. 1994), but generally the species reaches 40 to 60 mm (1.6-2.4 in) in shell height (a straight line measurement of the greatest distance between the umbo and the ventral margin), with a maximum size reported to be about 80 mm (3.2 in) in shell diameter (a straight line measurement of the greatest distance between the anterior and posterior margin) (Roe et al. -

Volume 2E - Revised Baseline Ecological Risk Assessment Hudson River Pcbs Reassessment
PHASE 2 REPORT FURTHER SITE CHARACTERIZATION AND ANALYSIS VOLUME 2E - REVISED BASELINE ECOLOGICAL RISK ASSESSMENT HUDSON RIVER PCBS REASSESSMENT NOVEMBER 2000 For U.S. Environmental Protection Agency Region 2 and U.S. Army Corps of Engineers Kansas City District Book 2 of 2 Tables, Figures and Plates TAMS Consultants, Inc. Menzie-Cura & Associates, Inc. PHASE 2 REPORT FURTHER SITE CHARACTERIZATION AND ANALYSIS VOLUME 2E- REVISED BASELINE ECOLOGICAL RISK ASSESSMENT HUDSON RIVER PCBs REASSESSMENT RI/FS CONTENTS Volume 2E (Book 1 of 2) Page TABLE OF CONTENTS ........................................................ i LIST OF TABLES ........................................................... xiii LIST OF FIGURES ......................................................... xxv LIST OF PLATES .......................................................... xxvi EXECUTIVE SUMMARY ...................................................ES-1 1.0 INTRODUCTION .......................................................1 1.1 Purpose of Report .................................................1 1.2 Site History ......................................................2 1.2.1 Summary of PCB Sources to the Upper and Lower Hudson River ......4 1.2.2 Summary of Phase 2 Geochemical Analyses .......................5 1.2.3 Extent of Contamination in the Upper Hudson River ................5 1.2.3.1 PCBs in Sediment .....................................5 1.2.3.2 PCBs in the Water Column ..............................6 1.2.3.3 PCBs in Fish .........................................7 -

2007 Ecology of Inshore Lizardfish
ECOLOGY OF INSHORE LIZARDFISH, Synodus foetens, IN THE NORTHERN GULF OF MEXICO by Sarah Ann Branson Jeffers B.S., The University of West Alabama, 2002 A thesis submitted to the Department of Biology College of Arts and Sciences The University of West Florida In partial fulfillment of the requirements for the degree of Master of Science 2007 The thesis of Sarah Ann Jeffers is approved: Dick A. Snyder, Ph.D., Committee Member Date Philip C. Darby, Ph.D., Committee Member Date William F. Patterson III, Ph.D., Committee Chair Date Accepted for the Department/Division: George L. Stewart, Ph.D., Chair Date Accepted for the College: Jane S. Halonen, Ph.D., Dean Date Accepted for the University: Richard S. Podemski, Ph.D., Dean of Graduate Studies Date ii ACKNOWLEDGMENTS I would like to specially thank my advisor, Dr. Will Patterson, for accepting me in his lab and supporting my thesis research. Thank you to the National Oceanographic and Atmospheric Administration, Louisiana Sea Grant, and UWF for funding this project. I would also like to thank the people that helped out with my project: Dave Wells for letting me tag along to collect fish, Beverly Barnett for all her advice and support, and Sean Creekmore and George Smith for all their hard work helping me process samples. I would like to thank all my labmates, Nicole Morris, Suzi Gibson, CeCe Lounder, and Dustin Addis for their help and advice. Thanks to Drew Hopper and Kendal Falana of the National Marine Fisheries Service (NMFS) for their help in the field and NMFS’s Robert Allman for advice on ageing lizardfish. -

Non-Genetic Inheritance and Changing Environments
NON-GENETIC INHERITANCE Mini-review • DOI: 10.2478/ngi-2013-0005 • NGI • 2012 • 38-50 Non-genetic inheritance and changing environments Abstract Santiago Salinas1*, Marc Mangel1,3, Climate change continues to impact species worldwide. Understanding Simon C. Brown2, Stephan B. Munch4 and predicting how populations will respond is of clear importance. Here, we review a mechanism by which populations may respond rapidly to these changes: Trans-Generational Plasticity (TGP). TGP exists when the 1Center for Stock Assessment Research, 3Dept. of Biology, University of Bergen, environment experienced by the parents affects the shape of the reaction University of California Santa Cruz, Bergen, 5020, Norway norm in their offspring; that is, the parental and offspring environments Santa Cruz, CA 95060, USA interact to determine the offspring phenotype. We survey 80 empirical 4 2Dept. of Ecology and Evolutionary Biology, Fisheries Ecology Division, studies from 63 species (32 orders, 9 phyla) that demonstrate TGP. Overall, University of California Santa Cruz, Southwest Fisheries Science Center, TGP is taxonomically widespread and present in response to environmental Santa Cruz, CA, 95060, USA Santa Cruz, CA, 95060, USA drivers likely to be impacted by climate change. Although many examples now exist, we also identify areas of research that could greatly improve our understanding of TGP. We conclude that TGP is sufficiently established both theoretically and empirically to merit study as a potential coping tactic against rapid environmental changes.