Business Report Vol.45
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S
Bank of Japan Working Paper Series Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S. Yoshihiko Hogen* [email protected] Ko Miura** [email protected] Koji Takahashi*** [email protected] No.17-E-8 Bank of Japan June 2017 2-1-1 Nihonbashi-Hongokucho, Chuo-ku, Tokyo 103-0021, Japan ***Research and Statistics Department (currently at the Monetary Affairs Department) *** Research and Statistics Department *** Research and Statistics Department (currently at the Financial System and Bank Examination Department) Papers in the Bank of Japan Working Paper Series are circulated in order to stimulate discussion and comments. Views expressed are those of authors and do not necessarily reflect those of the Bank. If you have any comment or question on the working paper series, please contact each author. When making a copy or reproduction of the content for commercial purposes, please contact the Public Relations Department ([email protected]) at the Bank in advance to request permission. When making a copy or reproduction, the source, Bank of Japan Working Paper Series, should explicitly be credited. Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S. Yoshihiko Hogeny Ko Miuraz Koji Takahashix June 2017 Abstract Focusing on the recent secular stagnation debate, this paper examines the role of large …rm dynamics as determinants of productivity ‡uctuations. We …rst show that idiosyncratic shocks to large …rms as well as entry, exit, and reallocation e¤ects account for 30 to 40 percent of productivity ‡uctuations in Japan and the U.S. -
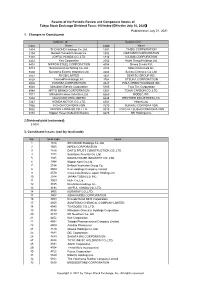
Published on July 21, 2021 1. Changes in Constituents 2
Results of the Periodic Review and Component Stocks of Tokyo Stock Exchange Dividend Focus 100 Index (Effective July 30, 2021) Published on July 21, 2021 1. Changes in Constituents Addition(18) Deletion(18) CodeName Code Name 1414SHO-BOND Holdings Co.,Ltd. 1801 TAISEI CORPORATION 2154BeNext-Yumeshin Group Co. 1802 OBAYASHI CORPORATION 3191JOYFUL HONDA CO.,LTD. 1812 KAJIMA CORPORATION 4452Kao Corporation 2502 Asahi Group Holdings,Ltd. 5401NIPPON STEEL CORPORATION 4004 Showa Denko K.K. 5713Sumitomo Metal Mining Co.,Ltd. 4183 Mitsui Chemicals,Inc. 5802Sumitomo Electric Industries,Ltd. 4204 Sekisui Chemical Co.,Ltd. 5851RYOBI LIMITED 4324 DENTSU GROUP INC. 6028TechnoPro Holdings,Inc. 4768 OTSUKA CORPORATION 6502TOSHIBA CORPORATION 4927 POLA ORBIS HOLDINGS INC. 6503Mitsubishi Electric Corporation 5105 Toyo Tire Corporation 6988NITTO DENKO CORPORATION 5301 TOKAI CARBON CO.,LTD. 7011Mitsubishi Heavy Industries,Ltd. 6269 MODEC,INC. 7202ISUZU MOTORS LIMITED 6448 BROTHER INDUSTRIES,LTD. 7267HONDA MOTOR CO.,LTD. 6501 Hitachi,Ltd. 7956PIGEON CORPORATION 7270 SUBARU CORPORATION 9062NIPPON EXPRESS CO.,LTD. 8015 TOYOTA TSUSHO CORPORATION 9101Nippon Yusen Kabushiki Kaisha 8473 SBI Holdings,Inc. 2.Dividend yield (estimated) 3.50% 3. Constituent Issues (sort by local code) No. local code name 1 1414 SHO-BOND Holdings Co.,Ltd. 2 1605 INPEX CORPORATION 3 1878 DAITO TRUST CONSTRUCTION CO.,LTD. 4 1911 Sumitomo Forestry Co.,Ltd. 5 1925 DAIWA HOUSE INDUSTRY CO.,LTD. 6 1954 Nippon Koei Co.,Ltd. 7 2154 BeNext-Yumeshin Group Co. 8 2503 Kirin Holdings Company,Limited 9 2579 Coca-Cola Bottlers Japan Holdings Inc. 10 2914 JAPAN TOBACCO INC. 11 3003 Hulic Co.,Ltd. 12 3105 Nisshinbo Holdings Inc. 13 3191 JOYFUL HONDA CO.,LTD. -

Itraxx Japan Series 35 Final Membership List March 2021
iTraxx Japan Series 35 Final Membership List March 2021 Copyright © 2021 IHS Markit Ltd T180614 iTraxx Japan Series 35 Final Membership List 1 iTraxx Japan Series 35 Final Membership List...........................................3 2 iTraxx Japan Series 35 Final vs. Series 34................................................ 5 3 Further information .....................................................................................6 Copyright © 2021 IHS Markit Ltd | 2 T180614 iTraxx Japan Series 35 Final Membership List 1 iTraxx Japan Series 35 Final Membership List IHS Markit Ticker IHS Markit Long Name ACOM ACOM CO., LTD. JUSCO AEON CO., LTD. ANAHOL ANA HOLDINGS INC. FUJITS FUJITSU LIMITED HITACH HITACHI, LTD. HNDA HONDA MOTOR CO., LTD. CITOH ITOCHU CORPORATION JAPTOB JAPAN TOBACCO INC. JFEHLD JFE HOLDINGS, INC. KAWHI KAWASAKI HEAVY INDUSTRIES, LTD. KAWKIS KAWASAKI KISEN KAISHA, LTD. KINTGRO KINTETSU GROUP HOLDINGS CO., LTD. KOBSTL KOBE STEEL, LTD. KOMATS KOMATSU LTD. MARUB MARUBENI CORPORATION MITCO MITSUBISHI CORPORATION MITHI MITSUBISHI HEAVY INDUSTRIES, LTD. MITSCO MITSUI & CO., LTD. MITTOA MITSUI CHEMICALS, INC. MITSOL MITSUI O.S.K. LINES, LTD. NECORP NEC CORPORATION NPG-NPI NIPPON PAPER INDUSTRIES CO.,LTD. NIPPSTAA NIPPON STEEL CORPORATION NIPYU NIPPON YUSEN KABUSHIKI KAISHA NSANY NISSAN MOTOR CO., LTD. OJIHOL OJI HOLDINGS CORPORATION ORIX ORIX CORPORATION PC PANASONIC CORPORATION RAKUTE RAKUTEN, INC. RICOH RICOH COMPANY, LTD. SHIMIZ SHIMIZU CORPORATION SOFTGRO SOFTBANK GROUP CORP. SNE SONY CORPORATION Copyright © 2021 IHS Markit Ltd | 3 T180614 iTraxx Japan Series 35 Final Membership List SUMICH SUMITOMO CHEMICAL COMPANY, LIMITED SUMI SUMITOMO CORPORATION SUMIRD SUMITOMO REALTY & DEVELOPMENT CO., LTD. TFARMA TAKEDA PHARMACEUTICAL COMPANY LIMITED TOKYOEL TOKYO ELECTRIC POWER COMPANY HOLDINGS, INCORPORATED TOSH TOSHIBA CORPORATION TOYOTA TOYOTA MOTOR CORPORATION Copyright © 2021 IHS Markit Ltd | 4 T180614 iTraxx Japan Series 35 Final Membership List 2 iTraxx Japan Series 35 Final vs. -

Ranking of Stocks by Market Capitalization(As of End of Jan.2018)
Ranking of Stocks by Market Capitalization(As of End of Jan.2018) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 244,072 2 8306 Mitsubishi UFJ Financial Group,Inc. 115,139 3 9437 NTT DOCOMO,INC. 105,463 4 9984 SoftBank Group Corp. 98,839 5 6861 KEYENCE CORPORATION 80,781 6 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 73,587 7 9433 KDDI CORPORATION 71,225 8 7267 HONDA MOTOR CO.,LTD. 69,305 9 8316 Sumitomo Mitsui Financial Group,Inc. 68,996 10 7974 Nintendo Co.,Ltd. 67,958 11 7182 JAPAN POST BANK Co.,Ltd. 66,285 12 6758 SONY CORPORATION 65,927 13 6954 FANUC CORPORATION 60,146 14 7751 CANON INC. 58,005 15 6902 DENSO CORPORATION 54,179 16 4063 Shin-Etsu Chemical Co.,Ltd. 53,624 17 8411 Mizuho Financial Group,Inc. 52,124 18 6594 NIDEC CORPORATION 52,025 19 9983 FAST RETAILING CO.,LTD. 51,647 20 4502 Takeda Pharmaceutical Company Limited 50,743 21 7201 NISSAN MOTOR CO.,LTD. 49,108 22 8058 Mitsubishi Corporation 48,497 23 2914 JAPAN TOBACCO INC. 48,159 24 6098 Recruit Holdings Co.,Ltd. 45,095 25 5108 BRIDGESTONE CORPORATION 43,143 26 6503 Mitsubishi Electric Corporation 42,782 27 9022 Central Japan Railway Company 42,539 28 6501 Hitachi,Ltd. 41,877 29 9020 East Japan Railway Company 41,824 30 6301 KOMATSU LTD. 41,162 31 3382 Seven & I Holdings Co.,Ltd. 39,765 32 6752 Panasonic Corporation 39,714 33 4661 ORIENTAL LAND CO.,LTD. 38,769 34 8766 Tokio Marine Holdings,Inc. -

State Street Global Advisors ESG Stock Exclusion List and Methodology in Relation to MSCI Produced Indices July 2021
1 State Street Global Advisors ESG Stock Exclusion List and Methodology in Relation to MSCI Produced Indices July 2021 Exclusion List for Q3-2021 Quarterly Index Rebalance Adani Enterprises Limited Jacobs Engineering Group Inc. Reinet Investments S.C.A. Adani Ports & Special Economic Zone Ltd. Japan Tobacco Inc. RLX Technology, Inc. Sponsored ADR Class A Airbus SE JBS S.A. Safran S.A. Altria Group Inc Korea Aerospace Industries, Ltd. Saudi Arabian Oil Company (Saudi Aramco) BAE Systems plc Korea Electric Power Corporation Saudi Basic Industries Corp. Boeing Company KT & G Corporation SK Inc. British American Tobacco p.l.c. Larsen & Toubro Infotech Ltd Smoore International Holdings Limited Canopy Growth Corporation Larsen & Toubro Ltd. Southern Copper Corporation China National Nuclear Power Co. Ltd. Class A Lockheed Martin Corporation Swedbank AB Class A China Northern Rare Earth (Group) High-Tech Co., Ltd. Metallurgical Corporation of China Ltd. Class A Swedish Match AB Class A China Petroleum & Chemical Corporation Class A MMC Norilsk Nickel PJSC Tata Consultancy Services Limited Teva Pharmaceutical Industries Limited Sponsored China Petroleum & Chemical Corporation Class H Motorola Solutions, Inc. ADR China Shipbuilding Industry Company Limited Class A MTN Group Limited Textron Inc. Danske Bank A/S Northrop Grumman Corporation Thales SA Tokyo Electric Power Company Holdings, Eastern Company Nutrien Ltd. Incorporated Elbit Systems Ltd Oil & Natural Gas Corp. Ltd. Toshiba Corporation Freeport-McMoRan, Inc. Pan American Silver Corp. Vale S.A. General Dynamics Corporation PetroChina Company Limited Class A Wal-Mart de Mexico SAB de CV Grupo Mexico S.A.B. de C.V. Class B PetroChina Company Limited Class H Walmart Inc. -

Daily Bulletin #1 VNL 2021 Bubble, Rimini, Italy
Daily Bulletin #1 VNL 2021 Bubble, Rimini, Italy Monday, May 24, 2021 Lausanne, 24 May 2021 VNL 2021 Bubble / Preliminary Inquiry Dear Participating Teams, We are happy to welcome you to the VNL 2021 Bubble in Rimini, Italy. With the scale of the event, we stress the importance of the Team Delegations to follow their individual activity schedules, and the requirement not to leave the hotel and / or competition complex, unless otherwise indicated. We note that members of some of the Team Delegations have not fully complied with the requirement, and ask first and foremost the Team Delegations to take responsibility for the enforcement of these measures. We truly thank your Team’s participation in the organized photoshoots and invite to visit the www.volleyballworld.com for the first photos available! Please note that the access to the Volleyball World TV platform shall be provided to each of your Team Delegation’s member shortly so you can follow the exciting volleyball action. We thank you for being part of the game! Kind Regards, Mr. Willy Paredes FIVB Technical Delegate FIVB Volleyball Nations League - Women O-2 Team registration BEL - Belgium Weight Height Highest reach [cm] National selections Shirt no Last name First name Shirt name Pos. Birthdate Club [kg] [cm] Spike Block WC OG Oth. Tot. 2 Van Sas Elise Van Sas S 01-Aug-1997 74 188 308 290 VC Oudegem - - 42 42 3 Herbots Britt Herbots OH 24-Sep-1999 63 182 312 292 Novara - - 84 84 4 Lemmens Nathalie Lemmens MB 12-Mar-1995 85 192 130 292 Paris - - 83 83 5 Guilliams Jodie Guilliams -

Annual Report FY2017
Corporate Information 048 Corporate Governance 058 History of the JT Group Japan Tobacco Inc. Annual Report FY2017 062 Regulation and Other Relevant Laws Year ended December 31, 2017 065 Litigation 066 Members of the Board, Audit Investment Operations & Analysis and Supervisory Board Members, leading and Executive Officers to sustainable 018 Industry Overview 067 Members of the JTI growth. 018 Tobacco Business Executive Committee 021 Pharmaceutical Business 067 Corporate Data 021 Processed Food Business 068 Investor Relations Activity 022 Review of Operations 069 Shareholder Information 022 International Tobacco Business 028 Japanese Domestic Tobacco Business 032 Global Tobacco Strategy 034 Pharmaceutical Business 038 Processed Food Business 040 Risk Factors Management 044 JT Group and Sustainability 046 Environmental, Social and 001 Performance Indicators Governance Initiatives 002 At a Glance 004 Consolidated Five-Year Financial Summary 006 Message from the Chairman and CEO Financial Information 008 CEO Business Review 010 Highlights (JT Group’s 2017) 071 Message from CFO 012 Management Principle, Strategic 072 Financial Review Framework and Resource Allocation 080 Consolidated Financial Statements 014 Business Plan 2018 086 Notes to Consolidated 015 Role and Target of Each Business Financial Statements 016 Performance Measures 139 Independent Auditor’s report 140 Glossary of Terms Management Performance Indicators Adjusted Operating Profit Dividend per Share 585.3 14 0 (JPY BN) (JPY) - 0.3% +7. 7 % Year-on-Year Change Year-on-Year Change - 0.6% Factsheets available at: Year-on-Year Change at Constant Exchange Rates https://www.jt.com/investors/results/annual_report/ Unless the context indicates otherwise, references in this Annual Report to ‘we’, ‘us’, In addition, these forward-looking statements are necessarily dependent upon ‘our’, ‘Japan Tobacco’, ‘JT Group’ or ‘JT’ are to Japan Tobacco Inc. -

International Companies with U.S. Branches
INTERNATIONAL COMPANIES WITH U.S. BRANCHES ® CLEMSON UNIVERSITY MICHELIN CAREER CENTER Company: Home Country: Type of Business: Royal Dutch/Shell Group Netherlands oil & gas Royal Dutch/Shell Group United Kingdom oil & gas BP United Kingdom oil & gas DaimlerChrysler Germany automobile Toyota Motor Japan automobile Mitsubishi Japan trading & distribution Mitsui & Co Japan trading & distribution Allianz Worldwide Germany insurance ING Group Netherlands diversified finance Volkswagen Group Germany automobile Sumitomo Japan trading & distribution Marubeni Japan trading & distribution Hitachi Japan electronic equipment Honda Motor Japan automobile AXA Group France insurance Sony Japan household durables Ahold Netherlands food & drug retail Nestle Switzerland food products Nissan Motor Japan automobile Credit Suisse Group Switzerland diversified finance Deutsche Bank Group Germany diversified finance BNP Paribas France bank Deutsche Telekom Germany telecom services Aviva United Kingdom insurance Generali Group Italy insurance Samsung Electronics South Korea semiconductor equip & prods Vodafone United Kingdom wireless telecom svcs Toshiba Japan electronic equipment ENI Italy oil & gas Unilever Netherlands food products Unilever United Kingdom food products Fortis Netherlands diversified finance France Telecom France telecom services UBS Switzerland diversified finance HSBC Group United Kingdom bank BMW-Bayerische Motor Germany automobile NEC Japan computers & peripherals Fujitsu Japan computers & peripherals Bayer HypoVereinsbank Germany bank -
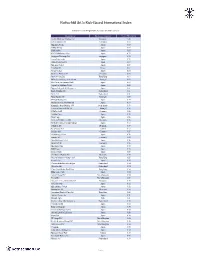
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Bribery & Corruption
Bribery & Corruption First Edition Contributing Editors: Jonathan Pickworth & Deborah Williams Published by Global Legal Group CONTENTS Preface Jonathan Pickworth & Deborah Williams, Dechert LLP Albania Silva Velaj & Sabina Lalaj, Boga & Associates 1 Argentina Marcelo den Toom & Mercedes de Artaza, M. & M. Bomchil 7 Australia Justin McDonnell, David Eliakim & Natalie Caton, King & Wood Mallesons 15 Bangladesh Dr. Kamal Hossain, Dr. Kamal Hossain and Associates 25 Belgium Joost Everaert, Nanyi Kaluma & Anthony Verhaegen, Allen & Overy LLP 30 Brazil Maurício Zanoide de Moraes, Caroline Braun & Daniel Diez Castilho, Zanoide de Moraes, Peresi & Braun Advogados Associados 36 Canada Mark Morrison, Paul Schabas & Michael Dixon, Blake, Cassels & Graydon LLP 44 Cayman Islands Martin Livingston & Brett Basdeo, Maples and Calder 52 China David Tiang, King & Wood Mallesons 60 Czech Republic Helena Hailichová & Eva Haisová, Johnson Šťastný Kramařík, advokátní kancelář, s.r.o. 70 France Julia Minkowski & Romain Fournier, Temime & Associés 78 Germany Sascha Kuhn, Simmons & Simmons LLP 87 Hong Kong Kyle Wombolt, Robert Hunt & Janice Tsang, Herbert Smith Freehills 95 India Siddharth Thacker, Mulla & Mulla & Craigie Blunt & Caroe 105 Indonesia Kyle Wombolt, Charles Ball & Narendra Adiyasa, Hiswara Bunjamin & Tandjung (HBT) in association with Herbert Smith Freehills 113 Ireland Megan Hooper & Heather Mahon, McCann FitzGerald 121 Italy Roberta Guaineri & Francesca Federici, Moro Visconti de Castiglione Guaineri 131 Japan Daiske Yoshida & Junyeon Park, Latham & Watkins 142 Mexico Edgar M. Anaya & Paula Nava González, Anaya Abogados Asociados, S.C. 151 Singapore Ing Loong Yang & Tina Wang, Latham & Watkins 160 South Africa Dave Loxton, Werksmans Attorneys 168 Spain Esteban Astarloa & Patricia Leandro, Uría Menéndez Abogados 176 Switzerland Grégoire Mangeat & Fanny Margairaz, Eversheds Ltd 186 Thailand Kyle Wombolt, Chinnawat Thongpakdee & Michelle Yu, Herbert Smith Freehills (Thailand) Ltd 197 Turkey Gönenç Gürkaynak & Ç. -

Bulletin#1 – 17Th Asian Sr. Women's Volleyball Championship
COMPETITION NAME (INCLUDING SEX AND CATEGORY) + LOGO Nom et emblème de la compétition (incluant sexe et catégorie) FEDERATION INTERNATIONALE DE VOLLEYBALL UNIFORM COLOURS O-1 Couleur de l'uniforme CITY : REGISTERED COLOURS / Couleurs enregistrées : Ville : (ONLY SHIRT COLOUR - seulement la couleur du maillot) D/j M/m Y/a TIME: H Min TEAMS : DATE : Heure: Equipes : 1 POOL/PHASE : Groupe/Phase : 2 MATCH SCHEDULE - Calendrier des matches TEAMS CHOICE - Choix des équipes ( 1 - 2 OR/ou 3) FIVB DECISION Team Team 1st CHOICE 2nd CHOICE Décision de la FIVB Equipe Equipe 1er Choix 2e Choix DATE : No. ABABABAB FIVB Technical Delegate : APPROVAL - Approbation : Délégué technique FIVB : FIVB Official form O-1 / January 2011 COMPETITION NAME (INCLUDING SEX AND CATEGORY) + LOGO Nom et emblème de la compétition (incluant sexe et catégorie) FEDERATION INTERNATIONALE DE VOLLEYBALL UNIFORM COLOURS O-1 Couleur de l'uniforme CITY : REGISTERED COLOURS / Couleurs enregistrées : Ville : (ONLY SHIRT COLOUR - seulement la couleur du maillot) D/j M/m Y/a TIME: H Min TEAMS : DATE : Heure: Equipes : 1 POOL/PHASE : Groupe/Phase : 2 MATCH SCHEDULE - Calendrier des matches TEAMS CHOICE - Choix des équipes ( 1 - 2 OR/ou 3) FIVB DECISION Team Team 1st CHOICE 2nd CHOICE Décision de la FIVB Equipe Equipe 1er Choix 2e Choix DATE : No. ABABABAB FIVB Technical Delegate : APPROVAL - Approbation : Délégué technique FIVB : FIVB Official form O-1 / January 2011 COMPETITION NAME (INCLUDING SEX AND CATEGORY) + LOGO Nom et emblème de la compétition (incluant sexe et catégorie) FEDERATION INTERNATIONALE DE VOLLEYBALL UNIFORM COLOURS O-1 Couleur de l'uniforme CITY : REGISTERED COLOURS / Couleurs enregistrées : Ville : (ONLY SHIRT COLOUR - seulement la couleur du maillot) D/j M/m Y/a TIME: H Min TEAMS : DATE : Heure: Equipes : 1 POOL/PHASE : Groupe/Phase : 2 MATCH SCHEDULE - Calendrier des matches TEAMS CHOICE - Choix des équipes ( 1 - 2 OR/ou 3) FIVB DECISION Team Team 1st CHOICE 2nd CHOICE Décision de la FIVB Equipe Equipe 1er Choix 2e Choix DATE : No. -

Welcome to the Delight World Vol.24
Welcome to the Delight World Vol.24 <Photo> Business Report CONTENTS Report on the Consolidated Financial Results for the Nine Months of the Fiscal Year Ending March 31, 2009 Illustrious Smokers: Hakushu Kitahara Feature: International Tobacco Business Interview with the President and CEO of Japan Tobacco International (JTI) International Tobacco Business Financial Results for 2008 (preliminary results) JTI Social Contribution Activities JT Thunders and JT Marvelous Fighting for their first championship win in the League! Bulletin Board JT Group Products - Roots “Aroma Black Hot Blend” now on sale - Roots “Fine Days! Low Sugar” now on sale - Lunch box series “Mini Spring Rolls” renewal - “Hot Noodles” renewal Share Handling Procedures – 1 – A Message from Management In the consolidated financial results for the nine months of the fiscal year ending March 31, 2009, net sales and EBITDA (operating income + depreciation and amortization) increased thanks to favorable growth in the international tobacco business as well as the addition of Gallaher and the Katokichi Group results to the consolidated performance. Meanwhile, operating income, ordinary income and net income decreased due in part to the start of amortization of goodwill in the international tobacco business following revisions to accounting standards. As for our earnings forecasts for the fiscal year ending March 31, 2009, we expect a record high in sales as a result of factors such as the favorable growth in the international tobacco business and the consolidation of Gallaher and the Katokichi Group. In addition, despite possible negative impact due to the strong yen on sales from yen-related currency exchange rates when consolidating overseas subsidiaries engaged in the international tobacco business, we project that continued sales volume growth will offset the negative foreign exchange effects with EBITDA hitting a record high.