Silver Hazards to Fish, Wildlife, and Invertebrates: a Synoptic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dermatology Eponyms – Sign –Lexicon (P)
2XU'HUPDWRORJ\2QOLQH Historical Article Dermatology Eponyms – sign –Lexicon (P)� Part 2 Piotr Brzezin´ ski1,2, Masaru Tanaka3, Husein Husein-ElAhmed4, Marco Castori5, Fatou Barro/Traoré6, Satish Kashiram Punshi7, Anca Chiriac8,9 1Department of Dermatology, 6th Military Support Unit, Ustka, Poland, 2Institute of Biology and Environmental Protection, Department of Cosmetology, Pomeranian Academy, Slupsk, Poland, 3Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan, 4Department of Dermatology, San Cecilio University Hospital, Granada, Spain, 5Medical Genetics, Department of Experimental Medicine, Sapienza - University of Rome, San Camillo-Forlanini Hospital, Rome, Italy, 6Department of Dermatology-Venerology, Yalgado Ouédraogo Teaching Hospital Center (CHU-YO), Ouagadougou, Burkina Faso, 7Consultant in Skin Dieseases, VD, Leprosy & Leucoderma, Rajkamal Chowk, Amravati – 444 601, India, 8Department of Dermatology, Nicolina Medical Center, Iasi, Romania, 9Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania Corresponding author: Piotr Brzezin′ski, MD PhD, E-mail: [email protected] ABSTRACT Eponyms are used almost daily in the clinical practice of dermatology. And yet, information about the person behind the eponyms is difficult to find. Indeed, who is? What is this person’s nationality? Is this person alive or dead? How can one find the paper in which this person first described the disease? Eponyms are used to describe not only disease, but also clinical signs, surgical procedures, staining techniques, pharmacological formulations, and even pieces of equipment. In this article we present the symptoms starting with (P) and other. The symptoms and their synonyms, and those who have described this symptom or phenomenon. Key words: Eponyms; Skin diseases; Sign; Phenomenon Port-Light Nose sign or tylosis palmoplantaris is widely related with the onset of squamous cell carcinoma of the esophagus. -

Culturing of Fathead Minnows (Pimephales Promelas) Supplement to Training Video
WHOLE EFFLUENT TOXICITY • TRAINING VIDEO SERIES • Freshwater Series Culturing of Fathead Minnows (Pimephales promelas) Supplement to Training Video U.S. Environmental Protection Agency Office of Wastewater Management Water Permits Division 1200 Pennsylvania Ave., NW Washington, DC 20460 EPA-833-C-06-001 December 2006 NOTICE The revision of this report has been funded wholly or in part by the Environmental Protection Agency under Contract EP-C-05-046. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. U.S. ENVIRONMENTAL PROTECTION AGENCY Culturing of Fathead Minnows (Pimephales promlas) Supplement to Training Video Foreword This report serves as a supplement to the video “Culturing of Fathead Minnows (Pimephales promelas)” (EPA, 2006a). The methods illustrated in the video and described in this report sup- port the methods published in the U.S. Environmental Protection Agency’s (EPA’s) Methods for Measuring the Acute Toxicity of Effluents to Freshwater and Marine Organisms, Fifth Edition (2002a) and Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, Fourth Edition (2002b), referred to as the Acute and Chronic Methods Manuals, respectively. The video and this report provide details on setting up and maintaining cultures based on the expertise of the personnel at the EPA’s Mid-Continent Ecology Division (MED) in Duluth, Minnesota (EPA-Duluth). More information can also be found in Guidelines for the Culture of Fathead Minnows (Pimephales promelas) for Use in Toxicity Tests (EPA, 1987). This report and its accompanying video are part of a series of training videos produced by EPA’s Office of Wastewater Management. -

Disease List for Aquaculture Health Certificate
Quarantine Standard for Designated Species of Imported/Exported Aquatic Animals [Attached Table] 4. Listed Diseases & Quarantine Standard for Designated Species Listed disease designated species standard Common name Disease Pathogen 1. Epizootic haematopoietic Epizootic Perca fluviatilis Redfin perch necrosis(EHN) haematopoietic Oncorhynchus mykiss Rainbow trout necrosis virus(EHNV) Macquaria australasica Macquarie perch Bidyanus bidyanus Silver perch Gambusia affinis Mosquito fish Galaxias olidus Mountain galaxias Negative Maccullochella peelii Murray cod Salmo salar Atlantic salmon Ameirus melas Black bullhead Esox lucius Pike 2. Spring viraemia of Spring viraemia of Cyprinus carpio Common carp carp, (SVC) carp virus(SVCV) Grass carp, Ctenopharyngodon idella white amur Hypophthalmichthys molitrix Silver carp Hypophthalmichthys nobilis Bighead carp Carassius carassius Crucian carp Carassius auratus Goldfish Tinca tinca Tench Sheatfish, Silurus glanis European catfish, wels Negative Leuciscus idus Orfe Rutilus rutilus Roach Danio rerio Zebrafish Esox lucius Northern pike Poecilia reticulata Guppy Lepomis gibbosus Pumpkinseed Oncorhynchus mykiss Rainbow trout Abramis brama Freshwater bream Notemigonus cysoleucas Golden shiner 3.Viral haemorrhagic Viral haemorrhagic Oncorhynchus spp. Pacific salmon septicaemia(VHS) septicaemia Oncorhynchus mykiss Rainbow trout virus(VHSV) Gadus macrocephalus Pacific cod Aulorhynchus flavidus Tubesnout Cymatogaster aggregata Shiner perch Ammodytes hexapterus Pacific sandlance Merluccius productus Pacific -

Guide to Policy & Practice Questions
OREGON BOARD OF CHIROPRACTIC EXAMINERS GUIDE TO POLICY & PRACTICE QUESTIONS 530 Center St NE, suite 620 Salem, OR 97301 (503) 378-5816 [email protected] Updated/Adopted: 9/17/2020 TABLE OF CONTENTS SECTION I ............................................................................................................................................................................................... 6 DEVICES, PROCEDURES, AND SUBSTANCES ............................................................................................................................... 6 DEVICES ................................................................................................................................................................ 6 BAX 3000 AND SIMILAR DEVICES................................................................................................................................................ 6 BIOPTRON LIGHT THERAPY ........................................................................................................................................................ 6 CPAP MACHINE, ORDERING ....................................................................................................................................................... 6 CTD MARK I MULTI-TORSION TRACTION DEVICE................................................................................................................... 6 DYNATRON 2000 ........................................................................................................................................................................... -

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -

450 (19) in Part C, the Following Chapters Are Added: "C.47 Fish
(19) In Part C, the following Chapters are added: "C.47 Fish, Early-life Stage Toxicity Test INTRODUCTION 1. This test method is equivalent to OECD test guideline (TG) 210 (2013). Tests with the early-life stages of fish are intended to define the lethal and sub-lethal effects of chemicals on the stages and species tested. They yield information of value for the estimation of the chronic lethal and sub-lethal effects of the chemical on other fish species. 2. Test guideline 210 is based on a proposal from the United Kingdom which was discussed at a meeting of OECD experts convened at Medmenham (United Kingdom) in November 1988 and further updated in 2013 to reflect experience in using the test and recommendations from an OECD workshop on fish toxicity testing, held in September 2010 (1). PRINCIPLE OF THE TEST 3. The early-life stages of fish are exposed to a range of concentrations of the test chemical dissolved in water. Flow-through conditions are preferred; however, if it is not possible semi-static conditions are acceptable. For details the OECD guidance document on aquatic toxicity testing of difficult substances and mixtures should be consulted (2). The test is initiated by placing fertilised eggs in test chambers and is continued for a species-specific time period that is necessary for the control fish to reach a juvenile life-stage. Lethal and sub-lethal effects are assessed and compared with control values to determine the lowest observed effect concentration (LOEC) in order to determine the (i) no observed effect concentration (NOEC) and/or (ii) ECx (e.g. -

Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal
ytology & f C H o is Prakash, et al., J Cytol Histol 2018, 9:2 l t a o n l o r DOI: 10.4172/2157-7099.1000501 g u y o J Journal of Cytology & Histology ISSN: 2157-7099 Research Article Open Access Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal Silver in Primate Vertebrates Pani Jyoti Prakash*1, Singh Royana2 and Pani Sankarsan3 1Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India 2Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttarpradesh, India 3Deapartment of Surgery, Institute of Medical Science and Research, Karjat, Bhivpuri, India *Corresponding author: Prakash PJ, Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India, Tel: 8433668356; E-mail: [email protected] Received date: February 21, 2018; Accepted date: March 12, 2018; Published date: March 16, 2018 Copyright: © 2018 Prakash PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Back ground: In this most recent, update global arena for consumers products most of the daily applications of bigger silver nano particles (20 to 100 nano meter range) are effected as anti-viral and anti-parasitic agents in clinical medicine and diagnosis which is a positive feedback. However, the major negative feedback of bigger size silver nano particles on human, animal and primate vertebrate body is multisystem teratogenicity focuses. Material and methods: This study was designed to investigate teratogenic effects of bigger size nano silver which is poly vinyl pyrollidone coated and sodium borohydride stabilized. -

A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient with Comorbid Schizoaffective Disorder
A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient With Comorbid Schizoaffective Disorder Sarah J. Schrauben, MD; Dhaval G. Bhanusali, MD; Stuart Sheets, MD; Animesh A. Sinha, MD, PhD Argyria is a rare cutaneous manifestation of silver is an elemental compound often found in drinking deposits in the skin, characterized by a grayish water, various sources in industry, and alternative blue discoloration, particularly in sun-exposed medicine compounds. Silver was first used medically areas. We report the case of a patient with a his- in 980 ad; it was believed to provide a means of tory of schizoaffective disorder and type 2 diabe- blood purification, to treat heart palpitations, and to tes mellitus who presented with argyria of the face serve as an adjunct treatment of epilepsy and tabes and neck. The patient had a history of ingesting dorsalis.1 In the 1900s, silver began being used as a colloidal silver proteins (CSPs) for approximately popular treatment of infections. However, reports of 10 years as a self-prescribed remedy for his medi- unsolicited side effects diminished its popularity as a cal conditions. CUTISmainstay solution for many ailments. Silver recently Colloidal silver protein has gained popularity has regained popularity, with an increase in Internet among patients who seek alternative medical ther- claims promoting the use of oral colloidal silver pro- apies. Argyria is the most predominant manifesta- teins (CSPs) as mineral supplements and as a way to tion of silver toxicity. It is unclear if our patient prevent and treat many diseases.2 began taking CSP becauseDo of his schizoaffectiveNot WeCopy present a patient with argyria as a conse- disorder or if silver toxicity may have induced quence of multiple forms of silver ingestion in an somatic delusions; however, it is important for attempt to treat his type 2 diabetes mellitus and physicians to have a thorough understanding of schizoaffective disorder. -
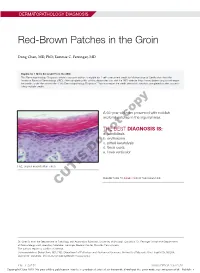
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

2021 Fish Suppliers
2021 Fish Suppliers A.B. Jones Fish Hatchery Largemouth bass, hybrid bluegill, bluegill, black crappie, triploid grass carp, Nancy Jones gambusia – mosquito fish, channel catfish, bullfrog tadpoles, shiners 1057 Hwy 26 Williamsburg, KY 40769 (606) 549-2669 ATAC, LLC Pond Management Specialist Fathead minnows, golden shiner, goldfish, largemouth bass, smallmouth bass, Rick Rogers hybrid bluegill, bluegill, redear sunfish, walleye, channel catfish, rainbow trout, PO Box 1223 black crappie, triploid grass carp, common carp, hybrid striped bass, koi, Lebanon, OH 45036 shubunkin goldfish, bullfrog tadpoles, and paddlefish (513) 932-6529 Anglers Bait-n-Tackle LLC Fathead minnows, rosey red minnows, bluegill, hybrid bluegill, goldfish and Kaleb Rodebaugh golden shiners 747 North Arnold Ave Prestonsburg, KY 606-886-1335 Andry’s Fish Farm Bluegill, hybrid bluegill, largemouth bass, koi, channel catfish, white catfish, Lyle Andry redear sunfish, black crappie, tilapia – human consumption only, triploid grass 10923 E. Conservation Club Road carp, fathead minnows and golden shiners Birdseye, IN 47513 (812) 389-2448 Arkansas Pondstockers, Inc Channel catfish, bluegill, hybrid bluegill, redear sunfish, largemouth bass, Michael Denton black crappie, fathead minnows, and triploid grass carp PO Box 357 Harrisbug, AR 75432 (870) 578-9773 Aquatic Control, Inc. Largemouth bass, bluegill, channel catfish, triploid grass carp, fathead Clinton Charlton minnows, redear sunfish, golden shiner, rainbow trout, and hybrid striped bass 505 Assembly Drive, STE 108 -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Health Hazard Evaluation Report 1981-0036-1023
Health Hazard HETA 81-036-1023 Evaluation ALASKA SMELTING &REFINING COMPANY Report WISILLAJ ALASKA PREFACE The Hazard Evaluations and Technical Assistance Branch of NIOSH conducts field investigations of possible health hazards in the workplace. These investigations are conducted under the authority of Section 20(a)(6) of the Occupational Safety and Health Act of 1970, 29 U.S .C. 669(a)(6} which authorizes the Secretary of Health and Human Services, following a written request from any employer or authorized representative of employees, to detennine whether any substance nonnally found in the place of employment has potentially toxic effects in such concentrations as used or found. The Hazard Evaluations and Technical Assistance Branch also provides, upon request, medical, nursing, and industrial hygiene technical and consultative assistance (TA) to Federal, state, and local agencies; labor; industry and other groups or individuals to control occupational health hazards and to prevent -related trauma and disease. i I l J Mention of company names or products does not constitute endorsement by the It National Institute for Occupational Safety and Health. ~ I f f HETA 81-036-1023 NIOSH INVESTIGATOR: December 1981 . Arvin G. Apol ALASKA SMELTING &REFINING Company Wisilla, Alaska I SUMMARY In November 1980, the National Institute for Occupational Safety and Health (NIOSH) received a request from the Alaska Smelting and Refining Company, Wisilla, Alaska, to determine if a health hazard existed as a result of the three workers' exposures to lead and silver fumes, dust, and chemicals found in the silver smelting and refining process. NIOSH conducted an environmental and medical evaluation on April 28-May 1, 1981.