Dyes and Dyeing Polar Versus Nonpolar Compounds SCIENTIFIC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
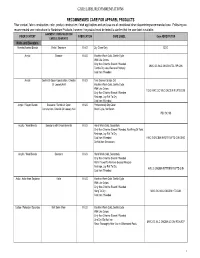
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Batik Workshop
Batik Workshop Batik is one of the "resist" processes for making designs on fabric, like Tie Dye, Shibori, Serti technique, etc., using wax on fabric to prevent dye from penetrating the cloth. Wax is applied to fabric, followed by dye, perhaps in many successive layers in complex Batiks. Batik is especially unique because the wax will crackle during handling, either intentionally or not. On subsequent dye baths, the crackles in the wax fill in with darker colors. Batik can be done with many types of dye or fabric paints & waxes on cottons, silks and other natural fabrics, particularly the finer weaves for detail work. "Faux" batik employs types of water soluble resists that are easier to remove than wax (and safer to work with for children), but never quite achieve that beautiful crackling. In this example we will be using Dharma Pigment Dyes and Soy Wax, on cotton, but can be adapted to other fabrics or dyes. The basic principles remain the same. Introduction to Batik Batik masters employ a process of repeated waxing and tub dyeing to achieve the final result. This method requires mastery of color mixing and over dyeing, as each layer of dye is applied over the last, producing a mixed color. After many different applications, the background usually comes out dark brown, black, or gray. The waxed areas remain the lighter shades produced by each individual application and combinations thereof. The Tub Dye technique is described below in more detail. An easier method of batik, especially for beginners, is the Paint-on method. This method has fewer steps and allows for great variations of color and shade without having to master the complicated blending of successive layers of color. -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Convertible Collar Construction
Convertible Collar Construction Directory Click any image to go to that section Yoke/Facing Options: Intro and Gallery By far the most common set-up for a The purpose of this introductory section is to convertible-collar shirt is that it has front facings feature and compare the range of other options and a yoke, and that these two details don’t touch, also, if less commonly, in use beyond this classic as in the example at right. one, before I proceed to work step-by-step through a handful of useful variants . Many other possible That is, the facings don’t extend far enough combinations, and of course, variations on the towards the shoulders at the neckline that they’ll ones here, are conceiveable and may suit your meet with or join to the fronts of the yoke layers. As project better, so feel free to experiment. a result, the yoke construction steps aren’t integrated into the collar steps and are completed, in front at least, before the collar is begun, so the options for using the yoke as a back facing are eliminated. The steps for this classic arrangement are described below in Variation #5, in the Front Facing Only category. Collar Insertion Options Step-By-Step No Yoke or Facings Required Front facings Only Front and Back Facings, or Yoke Used as Facing Variation 1: Collar Applied as Band Variation 3: Collar’s Back Neckline Edge-Stitched Variation 6: Back Facings 1 3 and Facings Secured at Shoulder Seams 6 Options: Options: 1. Edge-stitched neckline 2. -

Alum Mineral and the Importance for Textile Dyeing
Current Trends in Fashion Technology & Textile Engineering ISSN: 2577-2929 Mini-Review Curr Trends Fashion Technol Textile Eng Volume 3- Issue 4 - April 2018 Copyright © All rights are reserved by Ezatollah Mozaffari DOI: 10.19080/CTFTTE.2018.03.555619 Alum Mineral and the Importance for Textile Dyeing Ezatollah Mozaffari* and Bijan Maleki Imam khomeini international university, Qazvin, Iran Submission: Published: April 25, 2018 *Corresponding April author: 10, 2018; Email: Ezatollah Mozaffari, Imam khomeini International University, Qazvin, Iran, Tel: +9828-33901133; Abstract The importance of alum as a natural mordant in textile dyeing is explained. The history of alum mineral processing was reviewed to emphasise on the heritage knowledge inherited by current trends in fashion technology and textile engineering. The review will also demonstrate the conservative environmental preservation nature of alum mineral as mordant. The need for modern evaluation of natural dyes and mordants will be highlighted. Keywords: Alum; Mordant; Industrial heritage Introduction the calcined mass the calcined shale was barrowed to a series Alum was known as one of the most imperative components of stone leaching pits nearby with typical dimensions of 9 x of textile industry before the introduction of chemical dyes in 4.5 x 1.5m. Fresh liquid was added to the leaching tanks and the process repeated for several weeks. The waste solids were alum quarrying and trade in several geographical areas [1]. In the 1850s. Its significance could be explored when studying the literature, interesting notes on alum as a mordant for textile liquor from leaching rose to 1.12, indicating 12 tons of dissolved dyeing of yarn, cloth and leather in North America, China, Libya, eventually dug out and discarded. -

Batik Wax Instructions
Instructions Batik Wax Batik: A History Although its exact origin is uncertain, the earliest known batiks were discovered in Egyptian tombs dating back to the 4th century BCE. Wax-resist techniques were probably developed independently by disparate cultures throughout the ancient world. By the seventh century AD, patterning fabric using resists such as wax was a widespread practice throughout Asia and Africa and was perhaps most fully developed as an artform in Indonesia, where batik predates written records. By the thirteenth century, it became a highly respected art form and pastime for the women of Java and Bali, as recognizable motifs, patterns and colors became signifiers of one’s family and geographical area. Distinct styles and traditions proliferated and spread with the exchange of cultures through trade and exploration (see the “inland” and “coastal” batiks of Java, for instance — the two traditions couldn’t be more different). In the seventeenth century, as the world grew smaller, batik was introduced in Holland and other parts of Europe, where it became increasingly fashionable. Europeans and Americans traveling and living in the East encountered the ancient process and brought it back to their homelands — and spread it to colonies far away — where new traditions of batik branched out. Today, art schools across the United States and Europe offer batik courses as an essential part of their textile curricula. For more tips and techniques see www.jacquardproducts.com JACQUARD PRODUCTS Manufactured by Rupert, Gibbon & Spider, Inc. Healdsburg, CA 95448 | www.jacquardproducts.com | 800.442.0455 Batik Instructions Preparing and designing your fabric All new fabrics must be washed with hot soapy water, rinsed and dried to remove factory-applied sizings which may inhibit color penetration. -

The Maiwa Guide to NATURAL DYES W H at T H Ey a R E a N D H Ow to U S E T H E M
the maiwa guide to NATURAL DYES WHAT THEY ARE AND HOW TO USE THEM WA L NUT NATURA L I ND IG O MADDER TARA SYM PL O C OS SUMA C SE Q UO I A MAR IG O L D SA FFL OWER B U CK THORN LIVI N G B L UE MYRO B A L AN K AMA L A L A C I ND IG O HENNA H I MA L AYAN RHU B AR B G A LL NUT WE L D P OME G RANATE L O G WOOD EASTERN B RA ZIL WOOD C UT C H C HAMOM IL E ( SA PP ANWOOD ) A LK ANET ON I ON S KI NS OSA G E C HESTNUT C O C H I NEA L Q UE B RA C HO EU P ATOR I UM $1.00 603216 NATURAL DYES WHAT THEY ARE AND HOW TO USE THEM Artisans have added colour to cloth for thousands of years. It is only recently (the first artificial dye was invented in 1857) that the textile industry has turned to synthetic dyes. Today, many craftspeople are rediscovering the joy of achieving colour through the use of renewable, non-toxic, natural sources. Natural dyes are inviting and satisfying to use. Most are familiar substances that will spark creative ideas and widen your view of the world. Try experimenting. Colour can be coaxed from many different sources. Once the cloth or fibre is prepared for dyeing it will soak up the colour, yielding a range of results from deep jew- el-like tones to dusky heathers and pastels. -

Free Pattern Instructions Best Nest Organizer Basket
Fold 42" Quilted Hanging Basket Front Cut 1 on fold d l e on fo on e ont - plac fr Center Gather Quilted Hang ing Basket Gusset Cut 1 on fold -or- Cut 2 with seam allowance Free Pattern Instructions Pattern Free 21" Quilted Hanging Basket Back ld n fo n Cut 1 on fold o ce - pla ont er fr er t en C Layout Option 2: Layout From binding fabric, cut 2¼" wide cut strips bias equal fabric, to binding From 120". 118" to approximately seam to using a diagonal end, to end strips, binding Join Trim the seam open. then seams flat, Press bulk. reduce if ¼", needed. to allowances Careful meeting edges even. long in half, the binding Press press. the fabric as you stretch not to - the guidelines using a fabric marking pencil, pen or Mark, piece. pattern gathering the front for on as indicated Cut on fold Center base seam cutting line Fold • binding: the Create • • • 6" 3. 18" - Quilted Hanging Basket et Bask ing ang H Quilted Front ont Fr Cut 1 on fold fold on 1 t Cu d d l l e on fo e fo on e 42" t - plac t - plac ter fron ter fron ter n n Ce Ce Gather Gather et et ing Bask Bask ing ng ng Ha Ha Quilted Quilted t t e e k k s s a a B B g g in in g g n n a a H H d d te te il il u u Q Q ld ld t t se se s s Gu Gu k k c c a a B B d d ol ol f f n n o o ut 1 1 ut C C n fo n fo o o d d l l fo fo n n o o 1 1 t t u u C C r- r- -o -o ce ce ce ce n n wa wa allo allo m m a a th se se th wi wi 2 2 ut ut C C - pla - pla ont ont er fr er er fr t t en en C C Best Nest Organizer Basket Organizer Best Nest Layout Option 1: Layout yd. -

Textile Printing
TECHNICAL BULLETIN 6399 Weston Parkway, Cary, North Carolina, 27513 • Telephone (919) 678-2220 ISP 1004 TEXTILE PRINTING This report is sponsored by the Importer Support Program and written to address the technical needs of product sourcers. © 2003 Cotton Incorporated. All rights reserved; America’s Cotton Producers and Importers. INTRODUCTION The desire of adding color and design to textile materials is almost as old as mankind. Early civilizations used color and design to distinguish themselves and to set themselves apart from others. Textile printing is the most important and versatile of the techniques used to add design, color, and specialty to textile fabrics. It can be thought of as the coloring technique that combines art, engineering, and dyeing technology to produce textile product images that had previously only existed in the imagination of the textile designer. Textile printing can realistically be considered localized dyeing. In ancient times, man sought these designs and images mainly for clothing or apparel, but in today’s marketplace, textile printing is important for upholstery, domestics (sheets, towels, draperies), floor coverings, and numerous other uses. The exact origin of textile printing is difficult to determine. However, a number of early civilizations developed various techniques for imparting color and design to textile garments. Batik is a modern art form for developing unique dyed patterns on textile fabrics very similar to textile printing. Batik is characterized by unique patterns and color combinations as well as the appearance of fracture lines due to the cracking of the wax during the dyeing process. Batik is derived from the Japanese term, “Ambatik,” which means “dabbing,” “writing,” or “drawing.” In Egypt, records from 23-79 AD describe a hot wax technique similar to batik. -

(Cudrania Javanensis) Wood Extract Dyeing Quality on Silk Batik
Proceeding Indonesian Textile Conference (International Conference) 3rd Edition Volume 1 2019 http://itc.stttekstil.ac.id ISSN : 2356-5047 Effect of Different Solvent on Tegeran (Cudrania javanensis) Wood Extract Dyeing Quality on Silk Batik Vivin Atika 1 ,Tin Kusuma Arta 1, Dwi Wiji Lestari 1, Agus Haerudin 1and Aprilia Fitriani 1 1 Balai Besar Kerajinan dan Batik - Kementerian Perindustrian * Correspondence: [email protected]; Tel.: - Abstract: Tegeran (Cudrania javanensis) wood has been used as natural yellow dyes source for batik that traditionally obtained from extraction process by boiling in some amount of water. Tegeran dyes gives deep yellow color in cotton and silk batik with good fastness properties, but rarely reported about its result quality using different type of solvents in extracting process. Therefore, we conducted this research to find out how much different type of solvent in extracting process giving influence on dyeing result quality of silk batik. In this research has been carried out Tegeran wood extraction using four different solvent such as alcohol 70%, aquadest, acid/acetic acid solution pH 2.5 and alkaline/sodium hydroxide solution pH 12. The extract then used to dye batiked silk fabric. Tegeran wood extract coloring result in silk batik giving range colour from yellow to deep cream. The color strength values obtained were batik fabric dyed with Tegeran wood extract from alcohol 70% solvent 0.49, acid 0.57, aquadest 1.15, and alkaline 1.78. From color difference testing results, we obtain value of batik fabric colored with tegeran wood extract from alcohol 70 % L*=79.34, a*=6.37, b*=53.51; aquadest L*=87.91, a*=-0.69, b*=34.53; alkaline L*=76.06, a*=9.41, b*=14.76; and acid L*=77.70, a*=8.21, b*=36.72. -

Guide to Dyeing Yarn
presents Guide to Dyeing Yarn Learn How to Dye Yarn Using Natural Dyeing Techniques or some of us, the pleasure of using natural dyes is the connection it gives us with the earth, using plants and fungi and minerals from the environment in our Fhandmade projects. Others enjoy the challenge of finding, working with, and sometimes even growing unpredictable materials, then coaxing the desired hues. My favorite reason for using natural dyes is just plain lovely color. Sometimes subtle and always rich, the shades that skilled dyers achieve with natural dyestuffs are heart- breakingly lovely. No matter what inspires you to delve into natural dyes, this free eBook has some- thing for you. If you’re interested in connecting with the earth, follow Lynn Ruggles as she combines her gardening and fiber passions, or join Brighid’s Dyers as they harness alternative energy with solar dyeing. To test and improve your skills, begin with Dag- mar Klos’s thorough instructions. But whatever your reason, be sure to enjoy the range of natural colors on every page. One of Interweave’s oldest publications, Spin.Off inspires spinners to make beautiful yarn and find enchanting ways to use it. In addition to the quarterly magazine, we also host the spinning community spinningdaily.com, complete with blogs, forums, and free patterns. In our video workshop series, the living treasures of the spinning world share their knowledge. We’re devoted to bringing you the best spinning teachers, newest spinning techniques, and most inspiring ideas—right to your mailbox, your computer, and your very fingertips. -

Fashion Arts. Curriculum RP-54. INSTITUTION Ontario Dept
DOCUMENT RESUME ED 048 223 SP 007 137 TITLE Fashion Arts. Curriculum RP-54. INSTITUTION Ontario Dept. of Education, Toronto. PUB LATE 67 NOTE 34p. EDRS PRICE EDRS Price MF-$0.65 HC-$3.29 DESCRIPTORS Clothing Instruction, *Curriculum Guides, Distributive Education, *Grade 11, *Grade 12, *Hcme Economics, Interior Design, *Marketing, Merchandising, Textiles Instruction AESTRACT GRADES OR AGES: Grades 11 and 12. SUBJECT MATTER: Fashicn arts and marketing. ORGANIZATION AND PHkSTCAL APPEARANCE: The guide is divided into two main sections, one for fashion arts and one for marketing, each of which is further subdivided into sections fcr grade 11 and grade 12. Each of these subdivisions contains from three to six subject units. The guide is cffset printed and staple-todnd with a paper cover. Oi:IJECTIVE3 AND ACTIVITIES' Each unit contains a short list of objectives, a suggested time allotment, and a list of topics to he covered. There is only occasional mention of activities which can he used in studying these topics. INSTRUCTIONAL MATERIALS: Each unit contains lists of books which relate either to the unit as a whole or to subtopics within the unit. In addition, appendixes contain a detailed list of equipment for the fashion arts course and a two-page billiography. STUDENT A. ,'SSMENT:No provision. (RT) U $ DEPARTMENT OF hEALTH EOUCATION & WELFARE OFFICE OF THIS DOCUMENTEOUCATION HAS BEEN REPRO DUCED EXACT' VAS RECEIVED THE PERSON OR FROM INAnNO IT POINTSORGANIZATION ()RIG IONS STATED OF VIEW OR DO NUT OPIN REPRESENT OFFICIAL NECESSARILY CATION