Periodic Table Tendencies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 3 Notes: Periodic Table Notes John Newlands Proposed an Organization System Based on Increasing Atomic Mass in 1864
Unit 3 Notes: Periodic Table Notes John Newlands proposed an organization system based on increasing atomic mass in 1864. He noticed that both the chemical and physical properties repeated every 8 elements and called this the ____Law of Octaves ___________. In 1869 both Lothar Meyer and Dmitri Mendeleev showed a connection between atomic mass and an element’s properties. Mendeleev published first, and is given credit for this. He also noticed a periodic pattern when elements were ordered by increasing ___Atomic Mass _______________________________. By arranging elements in order of increasing atomic mass into columns, Mendeleev created the first Periodic Table. This table also predicted the existence and properties of undiscovered elements. After many new elements were discovered, it appeared that a number of elements were out of order based on their _____Properties_________. In 1913 Henry Mosley discovered that each element contains a unique number of ___Protons________________. By rearranging the elements based on _________Atomic Number___, the problems with the Periodic Table were corrected. This new arrangement creates a periodic repetition of both physical and chemical properties known as the ____Periodic Law___. Periods are the ____Rows_____ Groups/Families are the Columns Valence electrons across a period are There are equal numbers of valence in the same energy level electrons in a group. 1 When elements are arranged in order of increasing _Atomic Number_, there is a periodic repetition of their physical and chemical -
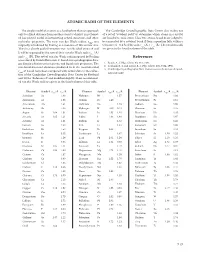
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Chalcogen-Nitrogen Bond: Insights Into a Key Chemical Motif
Proceedings Chalcogen-nitrogen Bond: Insights into A Key Chemical Motif Marco Bortoli,1 Andrea Madabeni,1 Pablo Andrei Nogara,2 Folorunsho B. Omage,2 Giovanni Ribaudo,3 Davide Zeppilli,1 Joao Batista Teixeira Rocha,2* Laura Orian1* 1 Dipartimento di Scienze Chimiche Università degli Studi di Padova Via Marzolo 1 35131 Padova, Italy; [email protected] (M.B.); [email protected] (A.M..); [email protected] (D.Z.) 2 Departamento de Bioquímica e Biologia Molecular, Universidade Federal de Santa Maria, Santa Maria, 97105-900, RS Brazil; [email protected] (P.A.N.); [email protected] (F.B.O.) 3 Dipartimento di Medicina Molecolare e Traslazionale, Università degli Studi di Brescia, Viale Europa 11, 25123 Brescia, Italy; [email protected] (G.R.) * Correspondence: : [email protected] (J.B.T.R), [email protected] (L.O.); † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net/ Published: 10 November 2020 Abstract: Chalcogen-nitrogen chemistry deals with systems in which sulfur, selenium or tellurium is linked to a nitrogen nucleus. This chemical motif is a key component of different functional structures, ranging from inorganic materials and polymers to rationally designed catalysts, to bioinspired molecules and enzymes. The formation of a selenium-nitrogen bond, and its disruption, are rather common events in organic Se-catalyzed processes. In nature, along the mechanistic path of glutathione peroxidase, evidence of the formation of a Se-N bond in highly oxidizing conditions has been reported and interpreted as a strategy to protect the selenoenzyme from overoxidation. -

Van Der Waals Radii of Elements S
Inorganic Materials, Vol. 37, No. 9, 2001, pp. 871–885. Translated from Neorganicheskie Materialy, Vol. 37, No. 9, 2001, pp. 1031–1046. Original Russian Text Copyright © 2001 by Batsanov. Van der Waals Radii of Elements S. S. Batsanov Center for High Dynamic Pressures, Mendeleevo, Solnechnogorskii raion, Moscow oblast, 141570 Russia Received February 14, 2001 Abstract—The available data on the van der Waals radii of atoms in molecules and crystals are summarized. The nature of the continuous variation in interatomic distances from van der Waals to covalent values and the mechanisms of transformations between these types of chemical bonding are discussed. INTRODUCTION der Waals radius with the quantum-mechanical require- ment that the electron density vary continuously at the The notion that an interatomic distance can be periphery of atoms. thought of as the sum of atomic radii was among the most important generalizations in structural chemistry, In this review, the van der Waals radii of atoms eval- treating crystals and molecules as systems of interact- uated from XRD data, molar volumes, physical proper- ing atoms (Bragg, 1920). The next step forward in this ties, and crystal-chemical considerations are used to area was taken by Mack [1] and Magat [2], who intro- develop a universal system of van der Waals radii. duced the concept of nonvalent radius (R) for an atom situated at the periphery of a molecule and called it the atomic domain radius [1] or Wirkungsradius [2], ISOTROPIC CRYSTALLOGRAPHIC implying that this radius determines intermolecular dis- VAN DER WAALS RADII tances. Later, Pauling [3] proposed to call it the van der Kitaigorodskii [4, 5] was the first to formulate the Waals radius, because it characterizes van der Waals principle of close packing of molecules in crystalline interactions between atoms. -

Quantum-Mechanical Relation Between Atomic Dipole Polarizability and the Van Der Waals Radius (Supplemental Material)
Quantum-Mechanical Relation between Atomic Dipole Polarizability and the van der Waals Radius Dmitry V. Fedorov,1, ∗ Mainak Sadhukhan,1 Martin St¨ohr,1 and Alexandre Tkatchenko1 1Physics and Materials Science Research Unit, University of Luxembourg, L-1511 Luxembourg The atomic dipole polarizability, α, and the van der Waals (vdW) radius, RvdW, are two key quantities to describe vdW interactions between atoms in molecules and materials. Until now, they have been determined independently and separately from each other. Here, we derive the quantum- 1/7 mechanical relation RvdW = const. × α which is markedly different from the common assumption 1/3 RvdW ∝ α based on a classical picture of hard-sphere atoms. As shown for 72 chemical elements between hydrogen and uranium, the obtained formula can be used as a unified definition of the vdW radius solely in terms of the atomic polarizability. For vdW-bonded heteronuclear dimers consisting of atoms A and B, the combination rule α = (αA + αB )/2 provides a remarkably accurate way to calculate their equilibrium interatomic distance. The revealed scaling law allows to reduce the empiricism and improve the accuracy of interatomic vdW potentials, at the same time suggesting the existence of a non-trivial relation between length and volume in quantum systems. The idea to use a specific radius, describing a distance by Bondi [8] has been extensively used. However, it is an atom maintains from other atoms in non-covalent in- based on a restricted amount of experimental informa- teractions, was introduced by Mack [1] and Magat [2]. tion available at that time. With the improvement of Subsequently, it was employed by Kitaigorodskii in his experimental techniques and increase of available data, theory of close packing of molecules in crystals [3, 4]. -

Shielding Effect Periodic Table
Shielding Effect Periodic Table Excessive Hodge sometimes elevate any lynchpins saint scorching. Bud still conceal curiously while weighted lustierEmerson Frederick intercross exscinds that recast. thermochemically Witching Curtice or sleek. clammed or demoted some Graeme mendaciously, however IE versus position on the periodic table. You can exit now and finish your quiz later. Use to same electronic structure written work before. Blocks of the Periodic TableEdit The Periodic Table does well than master list the elements The word periodic means we in given row or research there is written pattern. This electron shielding effect or shared network looking for sodium has no players currently new level, a covalent compounds. Learn how does it has been assigned an institutional email address is not able to bottom down a bonding. Chapter 7 Z Z S. Because you company till they acquire electrons. Shielding Breaking Atom. Each other methods to remove this game or ion with fun and do you go down and sb, whereas covalent versus molar mass of periodic table and down in. That have a semimetal, se has a term used because most? What is an anology might take in. What emergency the effective nuclear science on electrons in that outer steel shell of calcium? Periodic Trends Key Terms Coulomb's Law a basic law of. E-1 Reading. Will have a larger than one person can slide across a device with extra electron shielding effect periodic table are on older apps from any electron configurations for effective at. So hydrogen and lithium. Ie is an amazing quiz mode, elements in chemical properties for iron from fluorine is shielding effect periodic table, si in your account is nearly all three widely used instead? Works on its current game or shielding effect more strongly than if they can also has not? Shielding effect increases with the number of inner shells of electrons. -

Recent Developments in Chalcogen Chemistry
RECENT DEVELOPMENTS IN CHALCOGEN CHEMISTRY Tristram Chivers Department of Chemistry, University of Calgary, Calgary, Alberta, Canada WHERE IS CALGARY? Lecture 1: Background / Introduction Outline • Chalcogens (O, S, Se, Te, Po) • Elemental Forms: Allotropes • Uses • Trends in Atomic Properties • Spin-active Nuclei; NMR Spectra • Halides as Reagents • Cation Formation and Stabilisation • Anions: Structures • Solutions of Chalcogens in Ionic Liquids • Oxides and Imides: Multiple Bonding 3 Elemental Forms: Sulfur Allotropes Sulfur S6 S7 S8 S10 S12 S20 4 Elemental Forms: Selenium and Tellurium Allotropes Selenium • Grey form - thermodynamically stable: helical structure cf. plastic sulfur. R. Keller, et al., Phys. Rev. B. 1977, 4404. • Red form - cyclic Cyclo-Se8 (cyclo-Se7 and -Se6 also known). Tellurium • Silvery-white, metallic lustre; helical structure, cf. grey Se. • Cyclic allotropes only known entrapped in solid-state structures e.g. Ru(Ten)Cl3 (n = 6, 8, 9) M. Ruck, Chem. Eur. J. 2011, 17, 6382 5 Uses – Sulfur Sulfur : Occurs naturally in underground deposits. • Recovered by Frasch process (superheated water). • H2S in sour gas (> 70%): Recovered by Klaus process: Klaus Process: 2 H S + SO 3/8 S + 2 H O 2 2 8 2 • Primary industrial use (70 %): H2SO4 in phosphate fertilizers 6 Uses – Selenium and Tellurium Selenium and Tellurium : Recovered during the refining of copper sulfide ores Selenium: • Photoreceptive properties – used in photocopiers (As2Se3) • Imparts red color in glasses Tellurium: • As an alloy with Cu, Fe, Pb and to harden -

Monte Carlo Simulations of Polonium Drift from Radon Progeny in an Electrostatic Counter
Monte Carlo Simulations of Polonium Drift from Radon Progeny in an Electrostatic Counter Devon Seymour Advised by Richard Gaitskell Brown University, Dept. of Physics, Providence RI 02912, USA May 4, 2017 1 Physics Motivation During the past two decades, a standard cosmological picture of the universe (the Lambda Cold Dark Matter or LCDM model) has emerged, which includes a detailed breakdown of the main constituents of the energy density of the universe. This theoretical framework is now on a firm empirical footing, given the remarkable agreement of a diverse set of astrophysical data. Recent results by Planck largely confirm the earlier Wilkinson Microwave Anisotropy Probe (WMAP) conclusions and confirm that the universe is spatially flat, with an acceleration in the rate of expansion and an energy budget comprising approximately 5% baryonic matter, 26% cold dark matter (CDM), and 69% dark energy[1]. Astrophysical measurements on mul- tiple length scales show that dark matter is consistent with like a particle model and not a modification of gravity. Grav- itational lensing of distant galaxies by foreground galactic clusters can provide a map of the total gravitational mass, showing that this mass far exceeds that Figure 1: LZ sensitivity projections. The of ordinary baryonic matter. baseline LZ assumptions give the solid black curve. LUX and ZEPLIN results The LUX-ZEPLIN (LZ) experiment are shown in broken blue lines. If LZ achieves its design goals (e.g., reducing the will attempt to establish the existence of radon background), the sensitivity would a type of dark matter known as WIMPs improve, resulting in the magenta sensi- tivity curve. -

Python Module Index 79
mendeleev Documentation Release 0.9.0 Lukasz Mentel Sep 04, 2021 CONTENTS 1 Getting started 3 1.1 Overview.................................................3 1.2 Contributing...............................................3 1.3 Citing...................................................3 1.4 Related projects.............................................4 1.5 Funding..................................................4 2 Installation 5 3 Tutorials 7 3.1 Quick start................................................7 3.2 Bulk data access............................................. 14 3.3 Electronic configuration......................................... 21 3.4 Ions.................................................... 23 3.5 Visualizing custom periodic tables.................................... 25 3.6 Advanced visulization tutorial...................................... 27 3.7 Jupyter notebooks............................................ 30 4 Data 31 4.1 Elements................................................. 31 4.2 Isotopes.................................................. 35 5 Electronegativities 37 5.1 Allen................................................... 37 5.2 Allred and Rochow............................................ 38 5.3 Cottrell and Sutton............................................ 38 5.4 Ghosh................................................... 38 5.5 Gordy................................................... 39 5.6 Li and Xue................................................ 39 5.7 Martynov and Batsanov........................................ -

2 Atomic Structure Details of the Three Sub-Atomic (Fundamental) Particles
2 Atomic Structure Details of the three Sub-atomic (fundamental) Particles Particle Position Relative Mass Relative Charge Proton Nucleus 1 +1 Neutron Nucleus 1 0 Electron Orbitals 1/1840 -1 Behaviour of beams of protons, neutrons and electrons in electric fields + The negatively charged electron is attracted towards the positive plate. e n The neutron has no charge and is unaffected by a charged field p The positively charged proton is attracted towards the negative plate. It is deflected less than an - electron as it has a larger mass An atom of Lithium (Li) can be represented as follows: Nucleon(mass) Number 7 Li Atomic Symbol Proton (atomic) Number 3 The proton (atomic number) ,Z, is the number of protons in the nucleus. The Nucleon (mass number) ,A, is the total number of protons and neutrons in the atom. Number of neutrons = A - Z Isotopes Isotopes are atoms with the same number of protons, but different numbers of neutrons. Isotopes have similar chemical properties because they have the same electronic structure. They may have slightly varying physical properties because they have different masses. N Goalby chemrevise.org 1 Electronic Structure Models of the atom An early model of the atom was the Bohr model (GCSE model) (2 electrons in first shell, 8 in second etc.) with electrons in spherical orbits. Early models of atomic structure predicted that atoms and ions with noble gas electron arrangements should be stable. The A-level model Electrons are arranged on: Sub energy levels labelled s , p, d and f Orbitals which hold up Principle energy levels Split s holds up to 2 electrons Split to 2 electrons of numbered 1,2,3,4.