Biology EFFECTS of Mg2+, Cd2+, Cu2+ LOW CONCENTRATIONS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
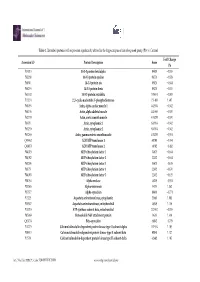
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

Contig Protein Description Symbol Anterior Posterior Ratio
Table S2. List of proteins detected in anterior and posterior intestine pooled samples. Data on protein expression are mean ± SEM of 4 pools fed the experimental diets. The number of the contig in the Sea Bream Database (http://nutrigroup-iats.org/seabreamdb) is indicated. Contig Protein Description Symbol Anterior Posterior Ratio Ant/Pos C2_6629 1,4-alpha-glucan-branching enzyme GBE1 0.88±0.1 0.91±0.03 0.98 C2_4764 116 kDa U5 small nuclear ribonucleoprotein component EFTUD2 0.74±0.09 0.71±0.05 1.03 C2_299 14-3-3 protein beta/alpha-1 YWHAB 1.45±0.23 2.18±0.09 0.67 C2_268 14-3-3 protein epsilon YWHAE 1.28±0.2 2.01±0.13 0.63 C2_2474 14-3-3 protein gamma-1 YWHAG 1.8±0.41 2.72±0.09 0.66 C2_1017 14-3-3 protein zeta YWHAZ 1.33±0.14 4.41±0.38 0.30 C2_34474 14-3-3-like protein 2 YWHAQ 1.3±0.11 1.85±0.13 0.70 C2_4902 17-beta-hydroxysteroid dehydrogenase 14 HSD17B14 0.93±0.05 2.33±0.09 0.40 C2_3100 1-acylglycerol-3-phosphate O-acyltransferase ABHD5 ABHD5 0.85±0.07 0.78±0.13 1.10 C2_15440 1-phosphatidylinositol phosphodiesterase PLCD1 0.65±0.12 0.4±0.06 1.65 C2_12986 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-1 PLCD1 0.76±0.08 1.15±0.16 0.66 C2_4412 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma-2 PLCG2 1.13±0.08 2.08±0.27 0.54 C2_3170 2,4-dienoyl-CoA reductase, mitochondrial DECR1 1.16±0.1 0.83±0.03 1.39 C2_1520 26S protease regulatory subunit 10B PSMC6 1.37±0.21 1.43±0.04 0.96 C2_4264 26S protease regulatory subunit 4 PSMC1 1.2±0.2 1.78±0.08 0.68 C2_1666 26S protease regulatory subunit 6A PSMC3 1.44±0.24 1.61±0.08 -

Serum Albumin OS=Homo Sapiens
Protein Name Cluster of Glial fibrillary acidic protein OS=Homo sapiens GN=GFAP PE=1 SV=1 (P14136) Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 Cluster of Isoform 3 of Plectin OS=Homo sapiens GN=PLEC (Q15149-3) Cluster of Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 (P68871) Vimentin OS=Homo sapiens GN=VIM PE=1 SV=4 Cluster of Tubulin beta-3 chain OS=Homo sapiens GN=TUBB3 PE=1 SV=2 (Q13509) Cluster of Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 (P60709) Cluster of Tubulin alpha-1B chain OS=Homo sapiens GN=TUBA1B PE=1 SV=1 (P68363) Cluster of Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTAN1 (Q13813-2) Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 Cluster of Spectrin beta chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTBN1 PE=1 SV=2 (Q01082) Cluster of Pyruvate kinase isozymes M1/M2 OS=Homo sapiens GN=PKM PE=1 SV=4 (P14618) Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 Clathrin heavy chain 1 OS=Homo sapiens GN=CLTC PE=1 SV=5 Filamin-A OS=Homo sapiens GN=FLNA PE=1 SV=4 Cytoplasmic dynein 1 heavy chain 1 OS=Homo sapiens GN=DYNC1H1 PE=1 SV=5 Cluster of ATPase, Na+/K+ transporting, alpha 2 (+) polypeptide OS=Homo sapiens GN=ATP1A2 PE=3 SV=1 (B1AKY9) Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 Dihydropyrimidinase-related protein 2 OS=Homo sapiens GN=DPYSL2 PE=1 SV=1 Cluster of Alpha-actinin-1 OS=Homo sapiens GN=ACTN1 PE=1 SV=2 (P12814) 60 kDa heat shock protein, mitochondrial OS=Homo -

Role of the HPRG Component of Striated Muscle AMP Deaminase in the Stability and Cellular Behaviour of the Enzyme
biomolecules Review Role of the HPRG Component of Striated Muscle AMP Deaminase in the Stability and Cellular Behaviour of the Enzyme Francesca Ronca * and Antonio Raggi Laboratory of Biochemistry, Department of Pathology, University of Pisa, via Roma 55, 56126 Pisa, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-050-2218-273; Fax: +39-050-2218-660 Received: 19 July 2018; Accepted: 20 August 2018; Published: 23 August 2018 Abstract: Multiple muscle-specific isoforms of the Zn2+ metalloenzyme AMP deaminase (AMPD) have been identified based on their biochemical and genetic differences. Our previous observations suggested that the metal binding protein histidine-proline-rich glycoprotein (HPRG) participates in the assembly and maintenance of skeletal muscle AMP deaminase (AMPD1) by acting as a zinc chaperone. The evidence of a role of millimolar-strength phosphate in stabilizing the AMPD-HPRG complex of both AMPD1 and cardiac AMP deaminase (AMPD3) is suggestive of a physiological mutual dependence between the two subunit components with regard to the stability of the two isoforms of striated muscle AMPD. The observed influence of the HPRG content on the catalytic behavior of the two enzymes further strengthens this hypothesis. Based on the preferential localization of HPRG at the sarcomeric I-band and on the presence of a Zn2+ binding motif in the N-terminal regions of fast TnT and of the AMPD1 catalytic subunit, we advance the hypothesis that the Zn binding properties of HPRG could promote the association of AMPD1 to the thin filament. Keywords: AMP deaminase (AMPD); histidine-proline-rich glycoprotein (HPRG); striated muscle; Troponin T (TnT) 1. -

Sugar, Uric Acid, and the Etiology of Diabetes and Obesity Richard J
PERSPECTIVES IN DIABETES Sugar, Uric Acid, and the Etiology of Diabetes and Obesity Richard J. Johnson,1,2 Takahiko Nakagawa,1,3 L. Gabriela Sanchez-Lozada,4 Mohamed Shafiu,5 Shikha Sundaram,6 Myphuong Le,1 Takuji Ishimoto,1 Yuri Y. Sautin,7 and Miguel A. Lanaspa1 The intake of added sugars, such as from table sugar (sucrose) For example, a high intake of fructose induces leptin re- and high-fructose corn syrup has increased dramatically in the sistance in rats (7). Fructose also encourages food intake last hundred years and correlates closely with the rise in obesity, due to stimulation of dopamine in the mesolimbic system metabolic syndrome, and diabetes. Fructose is a major compo- and effects on the hypothalamus (8,9). Food intake is also nent of added sugars and is distinct from other sugars in its ability stimulated by hepatic ATP depletion (10), which occurs in to cause intracellular ATP depletion, nucleotide turnover, and the animals and humans administered fructose (11). Fructose generation of uric acid. In this article, we revisit the hypothesis may also affect metabolic rate. A recent study in humans that it is this unique aspect of fructose metabolism that accounts documented a reduction in resting energy expenditure for why fructose intake increases the risk for metabolic syn- drome. Recent studies show that fructose-induced uric acid in overweight and obese subjects fed fructose but not generation causes mitochondrial oxidative stress that stimulates glucose (12). fat accumulation independent of excessive caloric intake. These studies challenge the long-standing dogma that “a calorie is just FRUCTOSE-INDUCED METABOLIC SYNDROME DOES NOT a calorie” and suggest that the metabolic effects of food may REQUIRE INCREASED ENERGY INTAKE matter as much as its energy content. -

Protein Symbol Protein Name Rank Metric Score 4F2 4F2 Cell-Surface
Supplementary Table 2 Supplementary Table 2. Ranked list of proteins present in anti-Sema4D treated macrophage conditioned media obtained in the GSEA analysis of the proteomic data. Proteins are listed according to their rank metric score, which is the score used to position the gene in the ranked list of genes of the GSEA. Values are obtained from comparing Sema4D treated RAW conditioned media versus REST, which includes untreated, IgG treated and anti-Sema4D added RAW conditioned media. GSEA analysis was performed under standard conditions in November 2015. Protein Rank metric symbol Protein name score 4F2 4F2 cell-surface antigen heavy chain 2.5000 PLOD3 Procollagen-lysine,2-oxoglutarate 5-dioxygenase 3 1.4815 ELOB Transcription elongation factor B polypeptide 2 1.4350 ARPC5 Actin-related protein 2/3 complex subunit 5 1.2603 OSTF1 teoclast-stimulating factor 1 1.2500 RL5 60S ribomal protein L5 1.2135 SYK Lysine--tRNA ligase 1.2135 RL10A 60S ribomal protein L10a 1.2135 TXNL1 Thioredoxin-like protein 1 1.1716 LIS1 Platelet-activating factor acetylhydrolase IB subunit alpha 1.1067 A4 Amyloid beta A4 protein 1.0911 H2B1M Histone H2B type 1-M 1.0514 UB2V2 Ubiquitin-conjugating enzyme E2 variant 2 1.0381 PDCD5 Programmed cell death protein 5 1.0373 UCHL3 Ubiquitin carboxyl-terminal hydrolase isozyme L3 1.0061 PLEC Plectin 1.0061 ITPA Inine triphphate pyrophphatase 0.9524 IF5A1 Eukaryotic translation initiation factor 5A-1 0.9314 ARP2 Actin-related protein 2 0.8618 HNRPL Heterogeneous nuclear ribonucleoprotein L 0.8576 DNJA3 DnaJ homolog subfamily -

Molecularly Imprinted Polymers for the Analysis of Protein Phosphorylation and the Role of Htra2/Omi Protein in Parkinson's Disease
Molecularly Imprinted Polymers for the Analysis of Protein Phosphorylation and the Role of HtrA2/Omi Protein in Parkinson's Disease by Jing Chen Dissertation Submitted to the Faculty of Chemistry and Biochemistry In Candidacy for the Degree of Doctor Rerum Naturalium (Dr. rer. nat) Accomplished at Medizinisches Proteom-Center Ruhr-Universität Bochum, Germany 03. 2015, Bochum Statement in Lieu of Oath I hereby declare that I have accomplished the thesis independently and did not submit to any other faculty or refer to more than the publications listed in the references. The digital figures contain only original data and no modification was added. There are altogether 5 identical copies of my dissertation. __________________________ Jing Chen I Referee: Prof. Dr. Katrin Marcus Co-referee: Dr. Dirk Wolters II Acknowledgement I would like to express my deep and sincere gratitude to Prof. Dr. Katrin Marcus, director of the Medizinische Proteom-Center, for her friendly invitation to the working group, for the great opportunity working in the interesting research field, for her dedication in supervising of my project execution and her unconditional help at the end of my Ph.D. I am very grateful to Dr. Dirk Wolters for his kind acceptance of attending and co- judging my dissertation. I owe my sincere gratitude to Dr. Stefan Helling, for his outstanding mentoring to this work. His valuable advice is deciding. Hadn’t for his endeavor in discussing and clearing my confusion at all times, I wouldn’t have managed to accomplish the work. I know Prof. Dr. Börje Sellergren, my collaboration partner at biomedical science in Malmö University, Sweden the longest. -

Functional Genomics Identifies AMPD2 As a New Prognostic Marker for Undifferentiated Pleomorphic Sarcoma
bioRxiv preprint doi: https://doi.org/10.1101/292805; this version posted March 31, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Orth et al. AMPD2 as prognostic marker in UPS Functional genomics identifies AMPD2 as a new prognostic marker for undifferentiated pleomorphic sarcoma Martin F. Orth1, Julia S. Gerke1, Thomas Knösel2, Annelore Altendorf-Hofmann3, Julian Musa1, Rebeca Alba Rubio1, Stefanie Stein1, Marlene Dallmayer1, Michaela C. Baldauf1, Aruna Marchetto1, Giuseppina Sannino1, Shunya Ohmura1, Jing Li1, Michiyuki Hakozaki4, Thomas Kirchner2,5,6, Thomas Dandekar7, Elke Butt8, Thomas G. P. Grünewald1,2,5,6,§ 1 Max-Eder Research Group for Pediatric Sarcoma Biology, Institute of Pathology, Faculty of Medicine, LMU Munich, Munich, Germany 2 Institute of Pathology, Faculty of Medicine, LMU Munich, Munich, Germany 3 Department of General, Visceral and Vascular Surgery, Jena University Hospital, Jena, Germany 4 Department of Orthopaedic Surgery, Fukushima Medical University School of Medicine, Fukushima, Japan 5 German Cancer Consortium (DKTK), partner site Munich, Germany 6 German Cancer Research Center (DKFZ), Heidelberg, Germany 7 Functional Genomics and Systems Biology Group, Department of Bioinformatics, Biocenter, Am Hubland, Würzburg, Germany 8 Institute for Experimental Biomedicine II, University Clinic of Würzburg, Würzburg, Germany -

32-2153: AMPD2 Recombinant Protein Description
9853 Pacific Heights Blvd. Suite D. San Diego, CA 92121, USA Tel: 858-263-4982 Email: [email protected] 32-2153: AMPD2 Recombinant Protein Adenosine Monophosphate Deaminase 2,Adenosine Monophosphate Deaminase 2 (Isoform L),EC Alternative Name 3.5.4.6,SPG63,AMP Deaminase Isoform L,AMP Deaminase 2,AMPD Isoform L,AMPD,PCH9,AMP : deaminase 2. Description Source : Escherichia Coli. AMPD2 Human Recombinant produced in E.Coli is a single, non-glycosylated polypeptide chain containing 667 amino acids (236-879 a.a) and having a molecular mass of 77.0kDa. AMPD2 is fused to a 23 amino acid His-tag at N-terminus & purified by proprietary chromatographic techniques. Adenosine Monophosphate Deaminase 2, also known as AMPD2 is significant in purine metabolism by converting AMP to IMP. AMPD2 which functions as a homotetramer, is one of the three AMP deaminases shown in mammals. More than a few transcript variants encoding different isoforms have been discovered for AMPD2. Product Info Amount : 10 µg Purification : Greater than 85% as determined by Analysis by SDS-PAGE. AMPD2 protein solution (0.25mg/ml) containing Phosphate buffered saline (pH7.4) and 10% Content : glycerol. Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of Storage condition : time. For long term storage it is recommended to add a carrier protein (0.1% HSA or BSA). Avoid multiple freeze-thaw cycles. Amino Acid : MGSSHHHHHH SSGLVPRGSH MGSDLLDAAK SVVRALFIRE KYMALSLQSF CPTTRRYLQQ LAEKPLETRT YEQGPDTPVSADAPVHPPAL EQHPYEHCEP STMPGDLGLG -

Fatty Acid Metabolism Driven Mitochondrial Bioenergetics Promotes Advanced Developmental Phenotypes in Human Induced Pluripotent Stem Cell Derived Cardiomyocytes
Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes Chrishan J.A. Ramachandra1,a,b, Ashish Mehta1,c, Philip Wonga,b,d,e*, K.P. Myu Mai Jaa, Regina Fritsche-Danielsonf, Ratan V. Bhatg, Derek J. Hausenloya,b,h,i,j, Jean-Paul Kovalikb and Winston Shima,b,k* aNational Heart Research Institute Singapore, National Heart Centre Singapore bCardiovascular & Metabolic Disorders Program, Duke-NUS Medical School, Singapore cPSC and Phenotyping Laboratory, Victor Chang Cardiac Research Institute, Sydney, Australia dDepartment of Cardiology, National Heart Centre Singapore eSchool of Materials Science and Engineering, Nanyang Technological University, Singapore fCardiovascular and Metabolic Disease Innovative Medicines and Early Development Unit, AstraZeneca Research and Development, Gothenburg, Sweden gStrategy and External Innovation Department, AstraZeneca, Gothenburg, Sweden hThe Hatter Cardiovascular Institute, University College London, United Kingdom iBarts Heart Centre, St Barthlomew’s Hospital, London, United Kingdom jYong Loo Lin School of Medicine, National University of Singapore kHealth and Social Sciences Cluster, Singapore Institute of Technology 1Both authors contributed equally Running title: Cardiomyocyte metabolism and bioenergetics *Corresponding authors: Philip Wong National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609 Email: [email protected]; Phone: +65 6704 8964; Fax: +65 6844 9053 Winston Shim -

Pathways of Adenine Nucleotide Catabolism in Erythrocytes
Pathways of adenine nucleotide catabolism in erythrocytes. F Bontemps, … , G Van den Berghe, H G Hers J Clin Invest. 1986;77(3):824-830. https://doi.org/10.1172/JCI112379. Research Article The exact pathway whereby the initial catabolism of the adenine nucleotides proceeds from AMP and the possibility of a recycling of adenosine were investigated in human erythrocytes. Adenine nucleotide catabolism, reflected by the production of hypoxanthine, is very slow under physiologic conditions and can be greatly increased by suppression of glucose or alkalinization of the medium. Experiments with inhibitors of adenosine deaminase and adenosine kinase demonstrated that under physiologic conditions the initial catabolism of AMP proceeds by way of a deamination of AMP, followed by dephosphorylation of inosine monophosphate, and that no recycling occurs between AMP and adenosine. Under glucose deprivation, approximately 75% of the 20-fold increase of the catabolism of the adenine nucleotides proceeded by way of a dephosphorylation of AMP followed by deamination of adenosine, and a small recycling of this nucleoside could be evidenced. Inhibition of adenosine transport showed that the dephosphorylation of AMP occurred intracellularly. When the incubation medium was alkalinized in the presence of glucose, the 15-fold increase in the conversion of AMP to hypoxanthine proceeded exclusively by way of AMP deaminase but a small recycling of adenosine could also be evidenced. The threefold elevation of intraerythrocytic inorganic phosphate (Pi) during glucose deprivation and its 50% decrease during alkalinization as well as experiments in which extracellular Pi was modified, indicate that the dephosphorylation of red […] Find the latest version: https://jci.me/112379/pdf Pathways of Adenine Nucleotide Catabolism in Erythrocytes F. -

Evidence for Sequential Expression of Multiple AMP Deaminase Isoforms During Skeletal Muscle Development RAINER MARQUETANT*, NALINI M
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 2345-2349, April 1987 Developmental Biology Evidence for sequential expression of multiple AMP deaminase isoforms during skeletal muscle development RAINER MARQUETANT*, NALINI M. DESAI*, RICHARD L. SABINA*t, AND EDWARD W. HOLMES*t tHoward Hughes Medical Institute Laboratories and *Departments of Medicine and Biochemistry, Duke University, Durham, NC 27710 Communicated by James B. Wyngaarden, December 22, 1986 ABSTRACT AMP deaminase (myoadenylate deaminase; MATERIALS EC 3.5.4.6) is an integral part of the myofibril in skeletal muscle, and this enzyme plays an important role in energy Male Sprague-Dawley LD strain rats were obtained from metabolism in this tissue. We report here the identification of Charles River Breeding Laboratories. The protease inhibi- three AMP deaminase isoforms during skeletal muscle devel- tors benzamidine, phenylmethylsulfonyl fluoride, leupeptin, opment in the rat. An embryonic isoform is expressed in the soybean trypsin inhibitor, pepstatin, a2-macroglobulin, developing hindlimb of the rat between 7 and 14 days of chymostatin, aprotinin, and antipain were obtained from gestation. This isoform is not unique to skeletal muscle or the Sigma. Staphylococcus aureus strain V8 proteinase was embryo as it is also expressed in many nonmuscle tissues of the obtained from Miles Laboratories. Electrophoresis reagents were supplied by Bio-Rad. Ampholytes were obtained from perinatal and adult rat. A perinatal isoform ofAMP deaminase LKB. [14C]AMP was purchased from New England Nuclear. that is restricted to skeletal muscle is produced 4-6 days before Nitrocellulose sheets were purchased from Schleicher & birth and persists for 2-3 weeks of postnatal life.