Role of the HPRG Component of Striated Muscle AMP Deaminase in the Stability and Cellular Behaviour of the Enzyme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
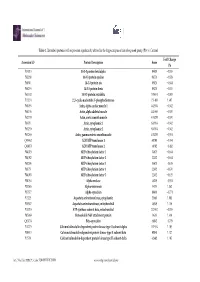
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

35 Disorders of Purine and Pyrimidine Metabolism
35 Disorders of Purine and Pyrimidine Metabolism Georges van den Berghe, M.- Françoise Vincent, Sandrine Marie 35.1 Inborn Errors of Purine Metabolism – 435 35.1.1 Phosphoribosyl Pyrophosphate Synthetase Superactivity – 435 35.1.2 Adenylosuccinase Deficiency – 436 35.1.3 AICA-Ribosiduria – 437 35.1.4 Muscle AMP Deaminase Deficiency – 437 35.1.5 Adenosine Deaminase Deficiency – 438 35.1.6 Adenosine Deaminase Superactivity – 439 35.1.7 Purine Nucleoside Phosphorylase Deficiency – 440 35.1.8 Xanthine Oxidase Deficiency – 440 35.1.9 Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency – 441 35.1.10 Adenine Phosphoribosyltransferase Deficiency – 442 35.1.11 Deoxyguanosine Kinase Deficiency – 442 35.2 Inborn Errors of Pyrimidine Metabolism – 445 35.2.1 UMP Synthase Deficiency (Hereditary Orotic Aciduria) – 445 35.2.2 Dihydropyrimidine Dehydrogenase Deficiency – 445 35.2.3 Dihydropyrimidinase Deficiency – 446 35.2.4 Ureidopropionase Deficiency – 446 35.2.5 Pyrimidine 5’-Nucleotidase Deficiency – 446 35.2.6 Cytosolic 5’-Nucleotidase Superactivity – 447 35.2.7 Thymidine Phosphorylase Deficiency – 447 35.2.8 Thymidine Kinase Deficiency – 447 References – 447 434 Chapter 35 · Disorders of Purine and Pyrimidine Metabolism Purine Metabolism Purine nucleotides are essential cellular constituents 4 The catabolic pathway starts from GMP, IMP and which intervene in energy transfer, metabolic regula- AMP, and produces uric acid, a poorly soluble tion, and synthesis of DNA and RNA. Purine metabo- compound, which tends to crystallize once its lism can be divided into three pathways: plasma concentration surpasses 6.5–7 mg/dl (0.38– 4 The biosynthetic pathway, often termed de novo, 0.47 mmol/l). starts with the formation of phosphoribosyl pyro- 4 The salvage pathway utilizes the purine bases, gua- phosphate (PRPP) and leads to the synthesis of nine, hypoxanthine and adenine, which are pro- inosine monophosphate (IMP). -

Purine Metabolism in Cultured Endothelial Cells
PURINE METABOLISM IN MAN-III Biochemical, Immunological, and Cancer Research Edited by Aurelio Rapado Fundacion Jimenez Diaz Madrid, Spain R.W.E. Watts M.R.C. Clinical Research Centre Harrow, England and Chris H.M.M. De Bruyn Department of Human Genetics University of Nijmegen Faculty of Medicine Nijmegen, The Netherlands PLENUM PRESS · NEW YORK AND LONDON Contents of Part Β I. PURINE METABOLISM PATHWAYS AND REGULATION A. De Novo Synthesis; Precursors and Regulation De Novo Purine Synthesis in Cultured Human Fibroblasts 1 R.B. Gordon, L. Thompson, L.A. Johnson, and B.T. Emmerson Comparative Metabolism of a New Antileishmanial Agent, Allopurinol Riboside, in the Parasite and the Host Cell 7 D. J. Nelson, S.W. LaFon, G.B. Elion, J.J. Marr, and R.L. Berens Purine Metabolism in Rat Skeletal Muscle 13 E. R. Tully and T.G. Sheehan Alterations in Purine Metabolism in Cultured Fibroblasts with HGPRT Deficiency and with PRPPP Synthetase Superactivity 19 E. Zoref-Shani and 0. Sperling Purine Metabolism in Cultured Endothelial Cells 25 S. Nees, A.L. Gerbes, B. Willershausen-Zönnchen, and E. Gerlach Determinants of 5-Phosphoribosyl-l-Pyrophosphate (PRPP) Synthesis in Human Fibroblasts 31 K.0, Raivio, Ch. Lazar, H. Krumholz, and M.A. Becker Xanthine Oxidoreductase Inhibition by NADH as a Regulatory Factor of Purine Metabolism 35 M.M. Jezewska and Z.W. Kaminski vii viii CONTENTS OF PART Β Β. Nucleotide Metabolism Human Placental Adenosine Kinase: Purification and Characterization 41 CM. Andres, T.D. Palella, and I.H. Fox Long-Term Effects of Ribose on Adenine Nucleotide Metabolism in Isoproterenol-Stimulated Hearts . -

Contig Protein Description Symbol Anterior Posterior Ratio
Table S2. List of proteins detected in anterior and posterior intestine pooled samples. Data on protein expression are mean ± SEM of 4 pools fed the experimental diets. The number of the contig in the Sea Bream Database (http://nutrigroup-iats.org/seabreamdb) is indicated. Contig Protein Description Symbol Anterior Posterior Ratio Ant/Pos C2_6629 1,4-alpha-glucan-branching enzyme GBE1 0.88±0.1 0.91±0.03 0.98 C2_4764 116 kDa U5 small nuclear ribonucleoprotein component EFTUD2 0.74±0.09 0.71±0.05 1.03 C2_299 14-3-3 protein beta/alpha-1 YWHAB 1.45±0.23 2.18±0.09 0.67 C2_268 14-3-3 protein epsilon YWHAE 1.28±0.2 2.01±0.13 0.63 C2_2474 14-3-3 protein gamma-1 YWHAG 1.8±0.41 2.72±0.09 0.66 C2_1017 14-3-3 protein zeta YWHAZ 1.33±0.14 4.41±0.38 0.30 C2_34474 14-3-3-like protein 2 YWHAQ 1.3±0.11 1.85±0.13 0.70 C2_4902 17-beta-hydroxysteroid dehydrogenase 14 HSD17B14 0.93±0.05 2.33±0.09 0.40 C2_3100 1-acylglycerol-3-phosphate O-acyltransferase ABHD5 ABHD5 0.85±0.07 0.78±0.13 1.10 C2_15440 1-phosphatidylinositol phosphodiesterase PLCD1 0.65±0.12 0.4±0.06 1.65 C2_12986 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-1 PLCD1 0.76±0.08 1.15±0.16 0.66 C2_4412 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma-2 PLCG2 1.13±0.08 2.08±0.27 0.54 C2_3170 2,4-dienoyl-CoA reductase, mitochondrial DECR1 1.16±0.1 0.83±0.03 1.39 C2_1520 26S protease regulatory subunit 10B PSMC6 1.37±0.21 1.43±0.04 0.96 C2_4264 26S protease regulatory subunit 4 PSMC1 1.2±0.2 1.78±0.08 0.68 C2_1666 26S protease regulatory subunit 6A PSMC3 1.44±0.24 1.61±0.08 -

Metabolism of Purines and Pyrimidines in Health and Disease
39th Meeting of the Polish Biochemical Society Gdañsk 16–20 September 2003 SESSION 6 Metabolism of purines and pyrimidines in health and disease Organized by A. C. Sk³adanowski, A. Guranowski 182 Session 6. Metabolism of purines and pyrimidines in health and disease 2003 323 Lecture The role of DNA methylation in cytotoxicity mechanism of adenosine analogues in treatment of leukemia Krystyna Fabianowska-Majewska Zak³ad Chemii Medycznej IFiB, Uniwersytet Medyczny, ul. Mazowiecka 6/8, 92 215 £ódŸ Changes in DNA methylation have been recognized tory effects of cladribine and fludarabine on DNA as one of the most common molecular alterations in hu- methylation, after 48 hr growth of K562 cells with the man neoplastic diseases and hypermethylation of drugs, are non-random and affect mainly CpG rich is- gene-promoter regions is one of the most frequent lands or CCGG sequences but do not affect sepa- mechanisms of the loss of gene functions. For this rea- rately-located CpG sequences. The analysis showed son, DNA methylation may be a tool for detection of that cladribine (0.1 mM) reduced the methylated early cell transformations as well as predisposition to cytosines in CpG islands and CCGG sequences to a sim- metastasis process. Moreover, DNA methylation seems ilar degree. The inhibition of cytosine methylation by to be a promissing target for new preventive and thera- fludarabine (3 mM) was observed mainly in CCGG se- peutic strategies. quences, sensitive to HpaII, but the decline in the meth- Our studies on DNA methylation and cytotoxicity ylated cytosine, located in CpG island was 2-fold lower mechanism of antileukemic drugs, cladribine and than that with cladribine. -

Determining HDAC8 Substrate Specificity by Noah Ariel Wolfson A
Determining HDAC8 substrate specificity by Noah Ariel Wolfson A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biological Chemistry) in the University of Michigan 2014 Doctoral Committee: Professor Carol A. Fierke, Chair Professor Robert S. Fuller Professor Anna K. Mapp Associate Professor Patrick J. O’Brien Associate Professor Raymond C. Trievel Dedication My thesis is dedicated to all my family, mentors, and friends who made getting to this point possible. ii Table of Contents Dedication ....................................................................................................................................... ii List of Figures .............................................................................................................................. viii List of Tables .................................................................................................................................. x List of Appendices ......................................................................................................................... xi Abstract ......................................................................................................................................... xii Chapter 1 HDAC8 substrates: Histones and beyond ...................................................................... 1 Overview ..................................................................................................................................... 1 HDAC introduction -

Serum Albumin OS=Homo Sapiens
Protein Name Cluster of Glial fibrillary acidic protein OS=Homo sapiens GN=GFAP PE=1 SV=1 (P14136) Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 Cluster of Isoform 3 of Plectin OS=Homo sapiens GN=PLEC (Q15149-3) Cluster of Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 (P68871) Vimentin OS=Homo sapiens GN=VIM PE=1 SV=4 Cluster of Tubulin beta-3 chain OS=Homo sapiens GN=TUBB3 PE=1 SV=2 (Q13509) Cluster of Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 (P60709) Cluster of Tubulin alpha-1B chain OS=Homo sapiens GN=TUBA1B PE=1 SV=1 (P68363) Cluster of Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTAN1 (Q13813-2) Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 Cluster of Spectrin beta chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTBN1 PE=1 SV=2 (Q01082) Cluster of Pyruvate kinase isozymes M1/M2 OS=Homo sapiens GN=PKM PE=1 SV=4 (P14618) Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 Clathrin heavy chain 1 OS=Homo sapiens GN=CLTC PE=1 SV=5 Filamin-A OS=Homo sapiens GN=FLNA PE=1 SV=4 Cytoplasmic dynein 1 heavy chain 1 OS=Homo sapiens GN=DYNC1H1 PE=1 SV=5 Cluster of ATPase, Na+/K+ transporting, alpha 2 (+) polypeptide OS=Homo sapiens GN=ATP1A2 PE=3 SV=1 (B1AKY9) Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 Dihydropyrimidinase-related protein 2 OS=Homo sapiens GN=DPYSL2 PE=1 SV=1 Cluster of Alpha-actinin-1 OS=Homo sapiens GN=ACTN1 PE=1 SV=2 (P12814) 60 kDa heat shock protein, mitochondrial OS=Homo -

Metabolomics Identifies Pyrimidine Starvation As the Mechanism of 5-Aminoimidazole-4-Carboxamide-1- Β-Riboside-Induced Apoptosis in Multiple Myeloma Cells
Published OnlineFirst April 12, 2013; DOI: 10.1158/1535-7163.MCT-12-1042 Molecular Cancer Cancer Therapeutics Insights Therapeutics Metabolomics Identifies Pyrimidine Starvation as the Mechanism of 5-Aminoimidazole-4-Carboxamide-1- b-Riboside-Induced Apoptosis in Multiple Myeloma Cells Carolyne Bardeleben1, Sanjai Sharma1, Joseph R. Reeve3, Sara Bassilian3, Patrick Frost1, Bao Hoang1, Yijiang Shi1, and Alan Lichtenstein1,2 Abstract To investigate the mechanism by which 5-aminoimidazole-4-carboxamide-1-b-riboside (AICAr) induces apoptosis in multiple myeloma cells, we conducted an unbiased metabolomics screen. AICAr had selective effects on nucleotide metabolism, resulting in an increase in purine metabolites and a decrease in pyrimidine metabolites. The most striking abnormality was a 26-fold increase in orotate associated with a decrease in uridine monophosphate (UMP) levels, indicating an inhibition of UMP synthetase (UMPS), the last enzyme in the de novo pyrimidine biosynthetic pathway, which produces UMP from orotate and 5-phosphoribosyl- a-pyrophosphate (PRPP). As all pyrimidine nucleotides can be synthesized from UMP, this suggested that the decrease in UMP would lead to pyrimidine starvation as a possible cause of AICAr-induced apoptosis. Exogenous pyrimidines uridine, cytidine, and thymidine, but not purines adenosine or guanosine, rescued multiple myeloma cells from AICAr-induced apoptosis, supporting this notion. In contrast, exogenous uridine had no protective effect on apoptosis resulting from bortezomib, melphalan, or metformin. Rescue resulting from thymidine add-back indicated apoptosis was induced by limiting DNA synthesis rather than RNA synthesis. DNA replicative stress was identified by associated H2A.X phosphorylation in AICAr-treated cells, which was also prevented by uridine add-back. -

TITLE Adenylate Kinase 2 Deficiency Causes NAD+ Depletion and Impaired Purine Metabolism During Myelopoiesis
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.05.450633; this version posted July 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. TITLE Adenylate Kinase 2 deficiency causes NAD+ depletion and impaired purine metabolism during myelopoiesis AUTHORS Wenqing Wang1, Andew DeVilbiss2, Martin Arreola1, Thomas Mathews2, Misty Martin-Sandoval2, Zhiyu Zhao2, Avni Awani1, Daniel Dever1, Waleed Al-Herz3, Luigi Noratangelo4, Matthew H. Porteus1, Sean J. Morrison2, Katja G. Weinacht1, * 1. Department of Pediatrics, Stanford University School of Medicine, Stanford, California 94305 USA 2. Children’s Research Institute, University of Texas Southwestern Medical Center, Dallas, Texas 75390 USA 3. Department of Pediatrics, Faculty of Medicine, Kuwait University, Safat, 13110 Kuwait 4. Laboratory of Clinical Immunology and Microbiology, National Institute of Health, BETHESDA MD 20814 USA * Corresponding author ABSTRACT Reticular Dysgenesis is a particularly grave from of severe combined immunodeficiency (SCID) that presents with severe congenital neutropenia and a maturation arrest of most cells of the lymphoid lineage. The disease is caused by biallelic loss of function mutations in the mitochondrial enzyme Adenylate Kinase 2 (AK2). AK2 mediates the phosphorylation of adenosine monophosphate (AMP) to adenosine diphosphate (ADP) as substrate for adenosine triphosphate (ATP) synthesis in the mitochondria. Accordingly, it has long been hypothesized that a decline in OXPHOS metabolism is the driver of the disease. The mechanistic basis for Reticular Dysgenesis, however, remained incompletely understood, largely due to lack of appropriate model systems to phenocopy the human disease. -

Adenosine Deaminase (ADA) Isoenzymes ADA1 and ADA2 in Biological Fluids
Eur Respir J 1997; 10: 2186–2187 Copyright ERS Journals Ltd 1997 DOI: 10.1183/09031936.97.10092186 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 CORRESPONDENCE Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2 in biological fluids To the Editor: readily obtained by measuring total ADA and the ADA2 which is not inhibited by 100 mmol·L-1 of added EHNA. We read with great interest the editorial by GAKIS [1] In fact, this method is being increasingly used by acquired about the extreme importance of the adenosine deami- immune deficiency syndrome (AIDS) researchers since nase (ADA) isoenzymes ADA1 and ADA2 on the some authors have reported a higher serum ADA activ- homeostasis of 2' deoxyadenosine and adenosine, espe- ity in human immunodeficiency virus (HIV)-1 positive cially when monocytes and macrophages are infected patients [4]. By the method we propose, some authors by intracellular microorganisms. Serum ADA activity have found that ADA2 isoenzyme activity is of con- is increased in various conditions such as liver disease, siderable prognostic value in AIDS and adult T-cell tuberculosis, typhoid, infective mononucleosis and cer- leukaemia (ATL) cases [5, 6]. tain malignancies, especially those of haematopoietic origin. The origin of serum ADA and the mechanisms by which serum activities are increased have not been References fully elucidated. 1. Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 Whatever the biological role of ADA1 and ADA2, it and ADA2: diagnostic and biological role. Eur Respir has been demonstrated that the presence (low or high) J 1996; 632–633. of these isoenzymes in biological fluids has diagnostic 2. -

STRIPE2 Encodes a Putative Dcmp Deaminase That Plays an Important Role in Chloroplast Development in Rice
Available online at www.sciencedirect.com ScienceDirect JGG Journal of Genetics and Genomics 41 (2014) 539e548 ORIGINAL RESEARCH STRIPE2 Encodes a Putative dCMP Deaminase that Plays an Important Role in Chloroplast Development in Rice Jing Xu a, Yiwen Deng a, Qun Li a, Xudong Zhu b,*, Zuhua He a,* a National Key Laboratory of Plant Molecular Genetics and National Center of Plant Gene Research, Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai 200032, China b China National Rice Research Institute, Hangzhou 31006, China Received 31 March 2014; revised 8 May 2014; accepted 9 May 2014 Available online 19 June 2014 ABSTRACT Mutants with abnormal leaf coloration are good genetic materials for understanding the mechanism of chloroplast development and chlorophyll biosynthesis. In this study, a rice mutant st2 (stripe2) with stripe leaves was identified from the g-ray irradiated mutant pool. The st2 mutant exhibited decreased accumulation of chlorophyll and aberrant chloroplasts. Genetic analysis indicated that the st2 mutant was controlled by a single recessive locus. The ST2 gene was finely confined to a 27-kb region on chromosome 1 by the map-based cloning strategy and a 5-bp deletion in Os01g0765000 was identified by sequence analysis. The deletion happened in the joint of exon 3 and intron 3 and led to new spliced products of mRNA. Genetic complementation confirmed that Os01g0765000 is the ST2 gene. We found that the ST2 gene was expressed ubiquitously. Subcellular localization assay showed that the ST2 protein was located in mitochondria. ST2 belongs to the cytidine deaminase-like family and possibly functions as the dCMP deaminase, which catalyzes the formation of dUMP from dCMP by deamination. -

Purine Nucleoside Phosphorylase, Or Adenosine Deaminase (Lesch-Nyhan Syndrome/Immunodeficiency) L
Proc. Natl. Acad. Sci. USA Vol. 75, No. 8, pp. 3722 -3726, August 1978 Biochemistry Purine metabolism in cultured human fibroblasts derived from patients deficient in hypoxanthine phosphoribosyltransferase, purine nucleoside phosphorylase, or adenosine deaminase (Lesch-Nyhan syndrome/immunodeficiency) L. F. THOMPSON*, R. C. WILLIS*, J. W. STOOPt, AND J. E. SEEGMILLER* * Department of Medicine, University of California, San Diego, La Jolla, California 92093; and t University Children's Hospital, Het Wilhelmina Kinderziekenhuis, Nieuwe Gracht 137, Utrecht, The Netherlands Contributed by J. Edwin Seegmiller, June 8, 1978 ABS14RACT Rates of purine synthesis de novo, as measured tive, high molecular weight form. Thus, either an increase in by the incorporation of [4C]formate into newly synthesized the concentration of PP-ribose-P or a decrease in the levels of purines, have been determined in cultured human fibroblasts derived from normal individuals and from patients deficient inhibitory nucleotides could potentially accelerate the rate of in adenosine deaminase, purine nucleoside phosporylase, or purine synthesis de novo and result in purine "overproduction." hypoxanthine phosphoribosyltransferase, three consecutive This communication concerns the study of purine metabolism enzymes of the purnne salvage pathway. All four types of cell in cultured human fibroblasts deficient in each of three en- lines are capable of incorporating [14C]formate into purines at zymes of the purine salvage pathway and attempts to define approximately the same