Deaths Due to Mixed Infections with Passalurus Ambiguus, Eimeria Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congressional Record-House. January 11
886 CONGRESSIONAL RECORD-HOUSE. JANUARY 11, CONFIRMATIONS. the Union Calendar, be referred back to that committee. Is there Executive nomination con.firmed by the Senate Decembe1· 20, 1.900. objection? [After a pause.] The Chair hears none. APPOINTMENT IN THE MARINE-HOSPITAL SERVICE. ACCOUNTS OF MARSHALS AND CLERKS OF DISTRICT COURTS IN THE TERRITORY OF UTAH. Benjamin S. Warren, of Alabama, to be an assis.tant surgeon in the Marine-Hospital Service of the United States. Mr. KING. Mr. Speaker, I ask unanimous consent for the present consideration of the bill (S. 5231) relating to the accounts Executive nominations con.firmed by the Senate Janua1'y 11, 1901. of United States marshals and clerks of the district courts of the UNITED STA.TES ATTORNEY. Territory of Utah. William G. Wheeler, of Wisconsin, to be attorney of the United _The SPEAKER. The gentleman from Utah asks unanimous States for the western district of Wisconsin. consent for the present consideration of the bill S. 5231, which is on the Speaker's table. POSTMASTER, The Clerk read as follows: David B. Rigdon, to be postmaster at Statesboro, Bu11och Be it enacted, etc., That the United States marshals and the clerks of the County, Ga. district courtg of the Territory of Utah prior to its admission to the Union as a State shall be held accountable only for fees earned in United States cases, in accordance with a decision of the Attorney-General dated Decem· · ber 2, 1891, and all unclosed accounts of such officers shall be settled and HOUSE OF REPRESENTATIVES. closed accordingly. -

Antony Van Leeuwenhoek, the Father of Microscope
Turkish Journal of Biochemistry – Türk Biyokimya Dergisi 2016; 41(1): 58–62 Education Sector Letter to the Editor – 93585 Emine Elif Vatanoğlu-Lutz*, Ahmet Doğan Ataman Medicine in philately: Antony Van Leeuwenhoek, the father of microscope Pullardaki tıp: Antony Van Leeuwenhoek, mikroskobun kaşifi DOI 10.1515/tjb-2016-0010 only one lens to look at blood, insects and many other Received September 16, 2015; accepted December 1, 2015 objects. He was first to describe cells and bacteria, seen through his very small microscopes with, for his time, The origin of the word microscope comes from two Greek extremely good lenses (Figure 1) [3]. words, “uikpos,” small and “okottew,” view. It has been After van Leeuwenhoek’s contribution,there were big known for over 2000 years that glass bends light. In the steps in the world of microscopes. Several technical inno- 2nd century BC, Claudius Ptolemy described a stick appear- vations made microscopes better and easier to handle, ing to bend in a pool of water, and accurately recorded the which led to microscopy becoming more and more popular angles to within half a degree. He then very accurately among scientists. An important discovery was that lenses calculated the refraction constant of water. During the combining two types of glass could reduce the chromatic 1st century,around year 100, glass had been invented and effect, with its disturbing halos resulting from differences the Romans were looking through the glass and testing in refraction of light (Figure 2) [4]. it. They experimented with different shapes of clear glass In 1830, Joseph Jackson Lister reduced the problem and one of their samples was thick in the middle and thin with spherical aberration by showing that several weak on the edges [1]. -
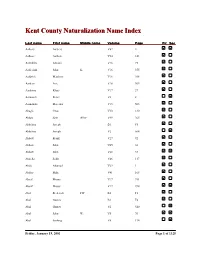
Kent County Naturalization Name Index, Aalbers, A
Kent County Naturalization Name Index Last name First name Middle name Volume Page Fir Sec Aalbers Aalbers V62 4 Aalbers Aalbert V24 141 Aalddriks Antonie V16 75 Aalderink John K. V16 355 Aaldrick Matthew V16 308 Aardem Arie V16 304 Aardema Klaas V17 27 Aarnouds Pieter V6 8 Aarnoudse Marenus V15 503 Abagis Chas V30 130 Abbas Sain Allez V49 265 Abbelma Joseph B1 F5 Abbelma Joseph V2 564 Abbott Frank V27 92 Abbott John V45 36 Abbott John V68 33 Abdella Salik V46 117 Abdo Ahamad V29 1 Abdoo Mike V41 168 Abeaf Moses V17 391 Abeaf Moses V17 394 Abel Frederick FW B1 F1 Abel Gustav B1 F4 Abel Gustav V2 540 Abel John W. V5 70 Abel Ludwig V8 134 Friday, January 19, 2001 Page 1 of 1325 Last name First name Middle name Volume Page Fir Sec Abella Salih V68 85 Abezi Albert V25 76 Aboabsee Theab V74 40 Aboasee Theab V18 150 Abood N. B1 F5 Abood Nemy V3 90 Abraham John V17 381 Abrahamson Charles Y. B7 200 Abram John B1 F1 Abramson Morris B1 F3 Abraursz Abram Peter V27 159 Abromaitis Louis V27 381 Abromaitis Louis V67 90 Absmaier Carl V77 4 Accardi Guiseppe V50 79 Acheson John V16 616 Achille Minciotti V51 142 Achtenhof Jakob V15 145 Achter Jan V17 200 Achterhof Henri B1 F1 Achterhof Johannes V15 500 Achterhof Matheus B1 F1 Achtjes John B7 107 Ackermann Joseph V15 282 Acton John C. B7 222 Adair David G. V15 335 Adair Joseph V15 335 Adalphson Emil V18 197 Friday, January 19, 2001 Page 2 of 1325 Last name First name Middle name Volume Page Fir Sec Adam Frickartz Heinrich V41 279 Adama Jelle V22 176 Adamawiczus Baltris V37 155 Adamczak Peter V38 245 Adamczyk Wladyslaw V35 291 Adams Edward John V24 70 Adams Frank B1 F2 Adams George W. -

Van Leeuwenhoek's Microscopes
46 Chapter 4 Chapter 4 Van Leeuwenhoek’s Microscopes While I am writing this letter, I have 8 or 10 magnifying glasses lying about, which have been mounted in silver by me; and although I never received any instruction in working in any metal with a hammer or a file, still I mount my glasses, and my tools have been fitted in such a way that master goldsmiths say that they cannot emulate me. In a letter comparing his ability to see sperm with the results claimed by Nicolaas Hartsoeker (see chapter 6), Antoni van Leeuwenhoek wrote to the Royal Society about how well he made his simple microscopes. It is frequently said that he invented the microscope, but this is not true. He improved the single-lens microscope enormously, but the manufacture and use of magnify- ing lenses began much earlier. The First Microscopes: A Brief History Magnifying lenses of one type or another have been in use for thousands of years. The oldest known lenses – made of polished crystals, usually quartz – date from 700 BC and were found in the Assyrian empire, and later in Egypt, Greece and Babylon. The Greek comic playwright Aristophanes (446–386 BC) wrote that burning glasses for the starting of fires were on sale in the shops of Athens. It is believed that the necessary magnification for the delicate work of cutting precious stones in antiquity was done using glass flasks filled with water. The Roman Stoic philosopher, Seneca (± 4 BC–65 AD), wrote that small letters, however small and unclear they may be, became large and clear when viewed through a glass bowl filled with water. -

Outlist 2010
Christopher Deal Bridget Hardy Gloria Leung Iris Ouyang Erica Shieh Brad Wergley Hiroshi Sasaki Viktor Kerney Jeremy Allen Elina Khodorkovsky Adam Wyatt Graduate Student, Master’s in Human Junior, Environmental Studies Freshman, Neuroscience Graduate Student, Linguistics Junior, Music Industry Senior, Cinema-Television Production Adjunct Assistant Professor, Masters Assistant Director for Student Leadership, Alumnus, B.A. in French, M.Ed. in PASA, Dedicated to LGBT youth that have taken Behavior (MHB) Program, Rossier School of Education Residential Education Alumnus, BA Fine Arts , 2010 Alumnus, B.A. International Relations and Anna Harley-Trochimczyk Ann Li Sara Parker Adam Siegel Marc Wetrich Tony Altimore Global Business, 2009 2004 Jackie DeLeon Senior, Chemical Engineering Junior, Business/IR Freshman, Business Administration in Graduate Student, Social Work Lisa Schweitzer Johnnathan Korver Alumnus, BS, 2001 Regina Lark Diane Yanez their own lives as a result of bullying, Sophomore, Film Production Alecs Harper Shuai Li Management Cinematic Arts Denice Wharton Associate Professor, School of Policy, Talent Acquisition Specialist, Career and Alumnus, Ph.D., 1999 Alumnus, Bachelors, Biology, 2006 Rebeca Delgado Sophomore, LNPS Graduate Student, Civil Engineering Aimee Sienkiewicz Senior, Political Science and Spanish Planning and Development Protective Services Cindy Ananias Sophomore, Writing for Film/Television Chris Passarelli Senior, Psychology Laura Isabel Serna Michael Kurland Alumnus, Health Promotion and Disease Radford Lathan harassment and campus homophobia. Keanna Harper Kevin Liang Senior, Political Science Gracie Wheelan Assistant Professor, School of Cinematic Academic Adviser, LAS College Prevention, 2009 Alumnus, Bachelor of Arts: Religion, Intl Campus Organization List Joshua DeMilta Sophomore, Business/Cinematic Arts Freshman, Biological Sciences Marie Paulo Erica Silva Senior, Critical Studies Arts, Critical Studies Debbie Ananias Relations, 2010 Sophomore, Broadcast Journalism Montana Harrington Alida Liberman Graduate Student, M.Ed. -

Research Highlights
[CLIENT] Dittrich1611 NT1510146 3 March 2017 Research Highlights GOALS Search for the death record of Barbara Dittrich’s alleged twin, Anežka. Find the birth records of Barbara’s other siblings in the Kladno parish records. Continue to extend the ancestry of Barbara Dittrich in available Czech records. PROGRESS Determined that Barbora Anežka was not a twin. Though unusual for Bohemia, many children in Kladno at that time had middle names. Reexamined the client’s information about Barbora’s siblings, and noted the town Libušín was listed for two of their births. Libušín was part of the Smečno parish, and that it was right outside of Kladno. It now appears that the family moved from Kladno to Libušín between 1892 and 1894. Discovered Barbora’s younger sister Agnes/Anežka’s marriage record in Canada to Antony Kraus. Also discovered her death record, which listed a second husband and her birth in Libušin. According to the client’s information, she had married Antony Kraus and had sons named Tony and Joe. We found her gravestone, and that of two of her children, Joseph and Agnes. Attempted to search the Libušín birth records for Barbora’s siblings but discovered that the relevant parish registers for Smečno are not yet digitized. The relevant book, Smečno 42, was also not available onsite. Sent an email requesting a search of the Libušín birth records after 1900 at the Libušín vital records office. Searched the available Kladno birth records from 1865-1870 for the births of Barbora’s parents. Their births were not found, but several more Dittrich and Veselý siblings for both parents were discovered in the process. -

Apollo 17 Index
Preparation, Scanning, Editing, and Conversion to Adobe Portable Document Format (PDF) by: Ronald A. Wells University of California Berkeley, CA 94720 May 2000 A P O L L O 1 7 I N D E X 7 0 m m, 3 5 m m, A N D 1 6 m m P H O T O G R A P H S M a p p i n g S c i e n c e s B r a n c h N a t i o n a l A e r o n a u t i c s a n d S p a c e A d m i n i s t r a t i o n J o h n s o n S p a c e C e n t e r H o u s t o n, T e x a s APPROVED: Michael C . McEwen Lunar Screening and Indexing Group May 1974 PREFACE Indexing of Apollo 17 photographs was performed at the Defense Mapping Agency Aerospace Center under the direction of Charles Miller, NASA Program Manager, Aerospace Charting Branch. Editing was performed by Lockheed Electronics Company, Houston Aerospace Division, Image Analysis and Cartography Section, under the direction of F. W. Solomon, Chief. iii APOLLO 17 INDEX 70 mm, 35 mm, AND 16 mm PHOTOGRAPHS TABLE OF CONTENTS Page INTRODUCTION ................................................................................................................... 1 SOURCES OF INFORMATION .......................................................................................... 13 INDEX OF 16 mm FILM STRIPS ........................................................................................ 15 INDEX OF 70 mm AND 35 mm PHOTOGRAPHS Listed by NASA Photograph Number Magazine J, AS17–133–20193 to 20375......................................... -

Lessons Learned from Success and Failure in Participatory Modeling
Portland State University PDXScholar Engineering and Technology Management Faculty Publications and Presentations Engineering and Technology Management 2-2019 Try, Try Again: Lessons Learned from Success and Failure in Participatory Modeling Eleanor J. Sterling American Museum of Natural History Moira Zellner The University of Illinois at Chicago Karen E. Jenni U.S. Geological Survey Kirsten Leong NOAA Pacific Islands Fisheries Science Center Pierre D. Glynn U.S. Geological Survey See next page for additional authors Follow this and additional works at: https://pdxscholar.library.pdx.edu/etm_fac Part of the Business Analytics Commons, and the Technology and Innovation Commons Let us know how access to this document benefits ou.y Citation Details Sterling, EJ, Zellner, M, Jenni, KE, Leong, K, Glynn, PD, BenDor, TK, Bommel, P, Hubacek, K, Jetter, AJ, Jordan, R, Olabisi, LS, Paolisso, M and Gray, S. 2019. Try, try again: Lessons learned from success and failure in participatory modeling. Elem Sci Anth, 7: 9. DOI: https://doi.org/10.1525/elementa.347 This Article is brought to you for free and open access. It has been accepted for inclusion in Engineering and Technology Management Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Authors Eleanor J. Sterling, Moira Zellner, Karen E. Jenni, Kirsten Leong, Pierre D. Glynn, Todd K. BenDor, Pierre Bommel, Klaus Hubacek, Antonie J. Jetter, Rebecca Jordan, Laura Schmitt Olabisi, Michael Paolisso, and Steven Gray This article is available at PDXScholar: https://pdxscholar.library.pdx.edu/etm_fac/163 Sterling, EJ, et al. -

Run Date: 08/30/21 12Th District Court Page
RUN DATE: 09/27/21 12TH DISTRICT COURT PAGE: 1 312 S. JACKSON STREET JACKSON MI 49201 OUTSTANDING WARRANTS DATE STATUS -WRNT WARRANT DT NAME CUR CHARGE C/M/F DOB 5/15/2018 ABBAS MIAN/ZAHEE OVER CMV V C 1/01/1961 9/03/2021 ABBEY STEVEN/JOH TEL/HARASS M 7/09/1990 9/11/2020 ABBOTT JESSICA/MA CS USE NAR M 3/03/1983 11/06/2020 ABDULLAH ASANI/HASA DIST. PEAC M 11/04/1998 12/04/2020 ABDULLAH ASANI/HASA HOME INV 2 F 11/04/1998 11/06/2020 ABDULLAH ASANI/HASA DRUG PARAP M 11/04/1998 11/06/2020 ABDULLAH ASANI/HASA TRESPASSIN M 11/04/1998 10/20/2017 ABERNATHY DAMIAN/DEN CITYDOMEST M 1/23/1990 8/23/2021 ABREGO JAIME/SANT SPD 1-5 OV C 8/23/1993 8/23/2021 ABREGO JAIME/SANT IMPR PLATE M 8/23/1993 2/16/2021 ABSTON CHERICE/KI SUSPEND OP M 9/06/1968 2/16/2021 ABSTON CHERICE/KI NO PROOF I C 9/06/1968 2/16/2021 ABSTON CHERICE/KI SUSPEND OP M 9/06/1968 2/16/2021 ABSTON CHERICE/KI NO PROOF I C 9/06/1968 2/16/2021 ABSTON CHERICE/KI SUSPEND OP M 9/06/1968 8/04/2021 ABSTON CHERICE/KI OPERATING M 9/06/1968 2/16/2021 ABSTON CHERICE/KI REGISTRATI C 9/06/1968 8/09/2021 ABSTON TYLER/RENA DRUGPARAPH M 7/16/1988 8/09/2021 ABSTON TYLER/RENA OPERATING M 7/16/1988 8/09/2021 ABSTON TYLER/RENA OPERATING M 7/16/1988 8/09/2021 ABSTON TYLER/RENA USE MARIJ M 7/16/1988 8/09/2021 ABSTON TYLER/RENA OWPD M 7/16/1988 8/09/2021 ABSTON TYLER/RENA SUSPEND OP M 7/16/1988 8/09/2021 ABSTON TYLER/RENA IMPR PLATE M 7/16/1988 8/09/2021 ABSTON TYLER/RENA SEAT BELT C 7/16/1988 8/09/2021 ABSTON TYLER/RENA SUSPEND OP M 7/16/1988 8/09/2021 ABSTON TYLER/RENA SUSPEND OP M 7/16/1988 8/09/2021 ABSTON -

Molecular Detection of Eimeria Stiedae in an Angora Rabbit
Etlik Vet Mikrobiyol Derg, 2015; 26 (2): 41-44 Case Report / Olgu Sunumu Molecular detection of Eimeria stiedae in an Angora rabbit Armağan Erdem ÜTÜK1, Fatma Çiğdem PİŞKİN2, İbrahim BALKAYA3, Mehmet Çağrı KARAKURUM4 1 Çukurova University, Faculty of Ceyhan Veterinary Medicine, Department of Parasitology, Adana, Turkey 2 Veterinary Control Central Research Institute, Parasitology and Bee Diseases Laboratory, Ankara, Turkey 3 Ataturk University, Faculty of Veterinary Medicine, Department of Parasitology, Erzurum, Turkey 4 Mehmet Akif Ersoy University, Faculty of Veterinary Medicine, Department of Internal Medicine, Burdur, Turkey Geliş Tarihi / Received: 04.09.2015, Kabul Tarihi / Accepted: 16.10.2015 Abstract: Hepatic coccidiosis caused by Eimeria stiedae, results in severe infection, outbreaks and deaths in young rabbits. Disease is diagnosed by time consuming methods like fecal examination, oocyst sporulation and necropsy. The aim of this study was to detect E.stiedae with species specific Polymerase Chain Reaction (PCR), without waiting oocyst sporulation and necropsy. Impression smears were prepared from liver nodules and stained with giemsa. Fecal samples were collected from the intestines and examined by centrifugal flotation with salt solution to detectEimeria spp. oocysts. Then oocysts were sporulated in 2.5% (w/v) aqueous potassium dichromate (K2Cr207). Primers specific to E.stiedae were used in PCR reaction. Eimeria spp. oocysts were detected after the examination of impression smears and bowel content. E.stiedae was diagnosed after the measurement of sporulated oocyst and PCR. At the end of the study, we think that; PCR will be very valuable for the rapid and true diagnosis of hepatic coccidiosis in rabbits. Key words: Angora rabbit, Eimeria stiedae, Molecular detection, Turkey. -

Thèse Coccidiose Du Poulet
اجلمهورية اجلزائرية الدميقراطية الشعبية République Algérienne Démocratique et Populaire وزارة التعليم العايل والبحث العلمي Ministère de l’Enseignement Supérieur et de la Recherche Scientifique Université Constantine 1 جامعة قسنطينة Institut des Sciences Vétérinaires 1 معهد العلوم البيطرية Thèse Présentée en vue de l’obtention du Doctorat en Sciences Vétérinaires Option : Pathologie aviaire Coccidiose du poulet : Etude pharmacologique et immunologique Présentée Par : DJEMAI Samir Membres du jury : Gamma 횪 휸 Omicron 횶 흄 Président BENCHEIKH ELFEGOUN Professeur Université de Constantine Mohammed Chérif Examinateur BENAKHLA Ahmed Professeur Université d’El-Tarf Examinateur AISSI Meriem Professeur École Nationale Supérieure Vétérinaire- Alger Examinateur ALLOUI Nadir Professeur Université de Batna Directeur de thèse MEKROUD Abdeslam Professeur Université de Constantine Année universitaire : 2016 / 2017 قَا َل َر ُسو َل ا ه َِّلل َص هَّل ا ه َُّلل عَلَْي ِه َو َس هَّل :« اللههُ هم انْ َف ْعِِن ِب َما عَله ْمتَِِن، َوعَِل ْمِِن َما يَْن َف ُعِِن، َوا ْرُز ْقِِن ِعلْ ًما تَ ْن َف ُعِِن ِب ِه » . ]السلسةل الصحيحة-حديث رمق 3151- ا أللباين[. Le Prophète- Que la prière d'Allah et Son salut soient sur lui- disait : « Ô Allah ! Fais- moi profiter de ce que Tu m’as appris, Apprends-moi ce qui m’apporte bénéfice et Accorde-moi une science par laquelle Tu Vas me faire profiter ». [Silsila Sahiha n°3151-Cheikh Al-Albani]. Prophet Muhammad-Peace be upon him- said : « Oh Allah ! Make useful for me what you have taught me, (and) teach me knowledge that will be useful to me and grant me such knowledge that will benefit me ». [Silsila Sahiha n°3151-Cheikh Al-Albani]. -

2019 Annual Report to the Community and Report on Philanthropy 2019 Annual Report to the Community and Report on Philanthropy
2019 Annual Report To the Community and Report on Philanthropy 2019 Annual Report To the Community and Report on Philanthropy Cover: Leading UH research on COVID-19, Grace McComsey, MD, Vice President of Research and Associate Chief Scientific Officer, UH Clinical Research Center, Rainbow Babies & Children's Foundation John Kennell Chair of Excellence in Pediatrics, and Division Chief of Infectious Diseases, UH Rainbow Babies and Children’s Hospital; and Robert Salata, MD, Chairman, Department of Medicine, STERIS Chair of Excellence in Medicine and and Master Clinician in Infectious Disease, UH Cleveland Medical Center, and Program Director, UH Roe Green Center for Travel Medicine and Global Health, are Advancing the Science of Health and the Art of Compassion. Photo by Roger Mastroianni The 2019 UH Annual Report to the Community and Report on Philanthropy includes photographs obtained before Ohio's statewide COVID-19 mask mandate. INTRODUCTION REPORT ON PHILANTHROPY 5 Letter to Friends 38 Letter to our Supporters 6 UH Statistics 39 A Gift for the Children 8 UH Recognition 40 Honoring the Philanthropic Spirit 41 Samuel Mather Society UH VISION IN ACTION 42 Benefactor Society 10 Building the Future of Health Care 43 Revolutionizing Men's Health 12 Defining the Future of Heart and Vascular Care 44 Improving Global Health 14 A Healing Environment for Children with Cancer 45 A New Game Plan for Sports Medicine 16 UH Community Highlights 48 2019 Endowed Positions 18 Expanding the Impact of Integrative Health 54 Annual Society 19 Beating Cancer with UH Seidman 62 Paying It Forward 20 UH Nurses: Advancing and Evolving Patient Care 63 Diamond Legacy Society 22 Taking Care of the Browns.