Cassin's Sparrow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Download This List As PDF Here
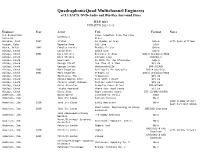
QuadraphonicQuad Multichannel Engineers of 5.1 SACD, DVD-Audio and Blu-Ray Surround Discs JULY 2021 UPDATED 2021-7-16 Engineer Year Artist Title Format Notes 5.1 Production Live… Greetins From The Flow Dishwalla Services, State Abraham, Josh 2003 Staind 14 Shades of Grey DVD-A with Ryan Williams Acquah, Ebby Depeche Mode 101 Live SACD Ahern, Brian 2003 Emmylou Harris Producer’s Cut DVD-A Ainlay, Chuck David Alan David Alan DVD-A Ainlay, Chuck 2005 Dire Straits Brothers In Arms DVD-A DualDisc/SACD Ainlay, Chuck Dire Straits Alchemy Live DVD/BD-V Ainlay, Chuck Everclear So Much for the Afterglow DVD-A Ainlay, Chuck George Strait One Step at a Time DTS CD Ainlay, Chuck George Strait Honkytonkville DVD-A/SACD Ainlay, Chuck 2005 Mark Knopfler Sailing To Philadelphia DVD-A DualDisc Ainlay, Chuck 2005 Mark Knopfler Shangri La DVD-A DualDisc/SACD Ainlay, Chuck Mavericks, The Trampoline DTS CD Ainlay, Chuck Olivia Newton John Back With a Heart DTS CD Ainlay, Chuck Pacific Coast Highway Pacific Coast Highway DTS CD Ainlay, Chuck Peter Frampton Frampton Comes Alive! DVD-A/SACD Ainlay, Chuck Trisha Yearwood Where Your Road Leads DTS CD Ainlay, Chuck Vince Gill High Lonesome Sound DTS CD/DVD-A/SACD Anderson, Jim Donna Byrne Licensed to Thrill SACD Anderson, Jim Jane Ira Bloom Sixteen Sunsets BD-A 2018 Grammy Winner: Anderson, Jim 2018 Jane Ira Bloom Early Americans BD-A Best Surround Album Wild Lines: Improvising on Emily Anderson, Jim 2020 Jane Ira Bloom DSD/DXD Download Dickinson Jazz Ambassadors/Sammy Anderson, Jim The Sammy Sessions BD-A Nestico Masur/Stavanger Symphony Anderson, Jim Kverndokk: Symphonic Dances BD-A Orchestra Anderson, Jim Patricia Barber Modern Cool BD-A SACD/DSD & DXD Anderson, Jim 2020 Patricia Barber Higher with Ulrike Schwarz Download SACD/DSD & DXD Anderson, Jim 2021 Patricia Barber Clique Download Svilvay/Stavanger Symphony Anderson, Jim Mortensen: Symphony Op. -

Inside Holy Cross - Inside
Inside Holy Cross - inside VOL. XXI, NO. 86 FRIDAY, FEBRUARY 6,1987 the independent student newspaper serving Notre Dame and Saint Mary’s Three platforms to run for SMC student body offices By MARILYN BENCHIK stated, “No experience necessary,” Assistant Saint Mary’s Editor and said, “ So, we’re just going to go for it.” Canidates for Saint Mary’s student Class officer canidates also attended body offices attended the second pre the Thursday night meeting. Running election information meeting Thursday for senior class officer positions are: night. Julie Bennett, Ana Cote, Patti Petro, Election hopefuls were required to at and Lorie Potenti. Potenti said they are tend only one of the two sessions. The not yet sure who will run for which po first pre-election meeting was held sition. Wednesday night. Sandy Cerimele, “ All four of us have had experience election commissioner, and Jeanne Hel in working with student government in ler, student body president, set the the last three years, and we feel we guidelines for campaigning have a great senior class. We want to procedures. make next year the most memorable,” The three platforms, each consisting Petro said. P of three canidates for the offices of Cote added, “Our experience is one president, vice president for student af of the most im portant factors to be con fairs, and vice president for academic sidered. We’ve already learned to deal affairs, are: Ann Rucker, Ann Reilly, with students’ problems and the issues and Ann Eckhoff; Sarah Cook, Janel which may arise.” Hamann, and Jill Hinterhalter; Eileen Canidates for the junior class posi Hetterich, Smith Hashagen, and Julie tions of president, vice president, sec Parrish. -

Politics Isn't Everything
Vol. 9 march 2017 — issue 3 iNSIDE: TOWNHALL TO NO ONE - POLITICS ISN’T EVERYTHING - COLD - why aren’t millennials eating pho - still drinking - BEST PUNK ALBUM EVER?? - ASK CREEPY HORSE - LOST BOYS OF THE RIGHT - misjumped - record reviews - concert calendar Town hall to no one Recently I was invited to attend a town hall meeting for our local representative to Congress, Rep. Bill Flores. In this day and age, you definitely want to have access to the person your district voted to send to 979Represent is a local magazine Washington and represent the interests of your home for the discerning dirtbag. area. 100 or so people turned out, submitted questions, gave passionate testimonials, aired grievances, ex- pressed outrage, even gave praise to Flores for a certain Editorial bored stance (though that was brief). Gay, straight, trans, white, other, Christian, Jew, Muslim, atheist, profession- Kelly Minnis - Kevin Still al, student, retired...a cross-section of the district’s diverse populace attended. Local police kept everything low key, local media showed to document the process. Art Splendidness Ivory is white, coal is black, water is wet...who cares, Katie Killer - Wonko The Sane right? This sort of thing happens all the time all over the country so what was so special about this event? Folks That Did the Other Shit For Us It was a townhall meeting with our congress- man...without the actual congressman in attendance. HENRY CLAYMORE - CREEPY HORSE - TIMOTHY MEATBALL He decided not to participate and instead flew to Mar-A- DANGER - MIKE L. DOWNEY - Jorge goyco - TODD HANSEN Lago to pull on Daddy Trump’s shirttail to beg of some EZEKIAL HENRY - Rented mule - HENRY ROWE - STARKNESS of the Orange Julius’s attention. -

Xtc Black Sea 5.1 Torrent Download Xtc Black Sea 5.1 Torrent Download
xtc black sea 5.1 torrent download Xtc black sea 5.1 torrent download. Newly mastered from the original stereo mix tapes, these three classic XTC albums are available again, but now in 200 gram vinyl form. The critically lauded SKYLARKING, APPLE VENUS (volume 1) and WASP STAR (Apple Venus volume 2) are awaiting your ears. Each with their complete running orders on 1 disc, the polarity corrected SKYLARKING sounds gorgeous and warm. AV1 has all it's orchestral and acoustic punch corralled into 1 platter, likewise WASP STAR, with it's slicing electric guitars. Cut by masterer Jason Mitchell at the world famous Loud Studios in the UK, everybody is saying how beautiful on the ears these sets are. Treat yourself, or a friend, today. 29th September 2017. BLACK SEA now in 5.1. THE 4TH XTC ALBUM - REMIXED & EXPANDED. Featuring new stereo mixes on CD & in Hi-Res Stereo & Surround mixes on Blu-Ray. Mixed by Steven Wilson from the original multi-track tapes & approved by Andy Partridge. Expanded booklet including new sleevenotes by Andy Partridge, Dave Gregory, Terry Chambers, Steve Lillywhite & Hugh Padgham. Blu-Ray also features a host of extras including: Original stereo mix & bonus tracks, additional songs from the album sessions in stereo & 5.1, 2017 instrumental mixes, many album tracks in demo form, the post-album single Take This Town from the soundtrack of the Times Square film, unissued single versions, mixes, additional tracks from singles of the period, solo demos by Andy Partridge & videos for Towers Of London, Generals & Majors & Respectable Street . 8th March 2017. -

Ogden Nasi, at Newark State Noted Poet and Humorist to Appear Wedn/Esday
Kean University Kean Digital Learning Commons Reflector 1950s Reflector 4-20-1959 The Reflector, Vol. 1, No. 19, April 20, 1959 Newark State College Follow this and additional works at: https://digitalcommons.kean.edu/reflector_1950s Recommended Citation Newark State College, "The Reflector, Vol. 1, No. 19, April 20, 1959" (1959). Reflector 1950s. 125. https://digitalcommons.kean.edu/reflector_1950s/125 This Book is brought to you for free and open access by the Reflector at Kean Digital Learning Commons. It has been accepted for inclusion in Reflector 1950s by an authorized administrator of Kean Digital Learning Commons. For more information, please contact [email protected]. eetor Volume 1, No. 18 N~WARK STATE COLLEGE, UNION, NEW JERSEY April 13, 1959 Ogden NaSI, At Newark State Noted Poet and Humorist To Appear Wedn/esday Coming to Newark State College on April 15th, as the third speaker in the Lecture Series sponsored by the Student Organiza_ tion, is the noted poet and humorist Ogden Nash. According to some critics, there are at least three sides to Ogden Nash:. The skylarking humorist, the cleft and experienced craftsman, and the remarkably serious social satirist. Using all these phases in his lecture, Mr. Nash will include the reading of some of his best • known poems from the New Yorker, The Saturday Evening Post. and other national magazines. A native New Yorker, Mr. Nash, except for a brief tenure as managing editor of the New Yorker in 1931, has concentrated for some twenty-five years on producing the light verses that have become associated with his name. -

Mechanicsburg Newsletter Spring 2016
U R O R A A S O C I A L Aurora Dawn EHABILITATION R CUMBERLAND & PERRY ERVICES S COUNTY NEWSLETTER SPRING 2016 Issue 6 Perry Council Arts Teams With New Bloomfield Aurora S P E C I A L Making a Difference in Our Community P O I N T S O F project a reality. She along with INTEREST: In a collaboration between Perry Council of the Art (PCCA), Au- the Perry County and Mechan- Star Wars Week rora Social Rehab, and Linda icsburg centers consumer met Billet, a mosaic artist from Hum- each morning at the PCCA Lan- Perry Council of the melstown PA a glass mosaic pro- dis House Art Room located at Arts Mosaic Project ject grant was awarded to the 67 N. 4th St. We had a difficult Jam Session council on behalf of persons with mental health challenges. The B.L.T. Kick Off final project will be displayed at the Newport Elementary school. Member Spotlight Linda Billet, (pictured right) is an outstanding artist who is not only INSIDE THIS passionate about her work, but ISSUE: her enthusiasm to teach others the joy of glass cutting was infec- Cover Story 1 tious. The 10 day step by step time envisioning the final prod- uct, but with Linda’s guidance Mechanicsburg 2 project was taught by Linda and and optimism she encouraged us News she helped the consumers to design, cut, shape, mold, grout every step of the way. She didn't Mechanicsburg 3 the sheets of glass in ways we leave anyone out of the produc- News never new possible. -

Teaching Notes by Susan La Marca Kate Mildenhall Is a Writer and Education Project Officer, Who Currently Works at the State Library of Victoria
Teaching Notes by Susan La Marca Kate Mildenhall is a writer and education project officer, who currently works at the State Library of Victoria. As a teacher, she has worked in schools, at RMIT University and has volunteered with Teachers Across Borders, delivering professional development to Khmer teachers in Cambodia. Skylarking is her debut novel. She lives with her husband and two young daughters in Hurstbridge, Victoria. TEACHING NOTES BY SUSAN LA MARCA Skylarking By Kate Mildenhall Abstract Kate and Harriet are best friends, growing up together on an isolated Australian cape in the 1880s. As daughters of the lighthouse keepers, the two girls share everything, until a fisherman, McPhail, arrives in their small community. When Kate witnesses the desire that flares between him and Harriet, she is torn by her feelings of envy and longing. But one moment in McPhail’s hut will change the course of their lives forever. Inspired by a true story, Skylarking is a stunning debut novel about friendship, love and loss, one that questions what it is to remember and how tempting it can be to forget. Prologue The first paragraph of the prologue and the first paragraph of the first chapter are both evocative descriptions of place. They conjure up an environment – both beautiful and harsh – with which many Australians can identify. Discuss how Mildenhall makes the reader feel with both of these descriptions. What aspects of the environment does she focus on? What makes the descriptions particularly Australian? This aspect of the book could be cleverly contrasted with poetry about the Australian environment. -

(Aimophila Cassinii) Status Assessment and Conservation Plan
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln US Fish & Wildlife Publications US Fish & Wildlife Service 2000 Cassin’s Sparrow (Aimophila cassinii) Status Assessment and Conservation Plan Janet M. Ruth U.S. Geological Survey, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usfwspubs Part of the Aquaculture and Fisheries Commons Ruth, Janet M., "Cassin’s Sparrow (Aimophila cassinii) Status Assessment and Conservation Plan" (2000). US Fish & Wildlife Publications. 170. https://digitalcommons.unl.edu/usfwspubs/170 This Article is brought to you for free and open access by the US Fish & Wildlife Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in US Fish & Wildlife Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Cassin’s Sparrow (Aimophila cassinii) Status Assessment and Conservation Plan Biological Technical Publication BTP-R6002-2000 Janet M. Ruth Research Ecologist U.S. Geological Survey Midcontinent Ecological Sicience Center 4512 Mc Murry Avenue Fort Collins, CO 80525-3400 970-226-9487 FAX 970-226-9230 [email protected] For additional copies or further information, contact: Nongame Migratory Bird Coordinator, Region 6 U.S. Fish & Wildlife Service P.O. Box 25486 Denver Federal Center Denver, CO 80225 March 2000 i Recommended citation: Ruth, J.M. 2000. Cassin’s Sparrow (Aimophila cassinii) status assessment and conservation plan. Biological Technical Publication BTP-R6002-1999. U.S. Department of the Interior, Fish and Wildlife Service, Denver, CO. ii U.S. Fish and Wildlife Service - Status Assessment and Conservation Plan Cassin’s Sparrow - March 2000 Table of Contents TABLE OF CONTENTS TABLE OF CONTENTS ........................................................... -

Assessment of Grassland Ecosystem Conditions in the Southwestern
Michele Merola-Zwartjes Chapter 4: Birds of Southwestern Grasslands: Status, Conservation, and Management Status and Conservation: are some of the primary factors that have influenced the diversity of species found here. The relative lack Introduction_______________________ of structural heterogeneity in grassland habitats has In the Southwestern United States, the grassland also played an important role in determining species avifauna is collectively composed of a mixture of spe- composition, as the lack of shrubs or trees eliminates cies found primarily in desert grasslands, shortgrass a variety of potential ecological niches for birds to steppe, wet meadows, and alpine tundra (as used here, exploit. This structural simplicity has resulted in an desert grasslands incorporate both arid grasslands avifauna that tends to be characterized by specialists, and desert shrub grasslands). Of these habitats, species that have evolved within those few, specific desert grasslands and shortgrass steppe are the niches available. Wilson’s phalarope, for example, is most extensive and support the greatest number of a wetland species that occurs only locally where water grassland bird species. Desert grasslands are patchily is available in the grassland landscape. Some spe- distributed across the southern halves of New Mexico cies, including burrowing owls, are highly dependent and Arizona, and shortgrass steppe is a component of upon active prairie dog towns, while others such as the Great Plains system that in the Southwest region Baird’s sparrow coevolved with grazing ungulates and extends across the eastern half of New Mexico into the consequently seek out habitats with a mosaic of grass panhandles of Texas and Oklahoma. Alpine tundra and heights. -

Chanting up Zion: Reggae As Productive Mechanism for Repatriated Rastafari In
Chanting up Zion: Reggae as Productive Mechanism for Repatriated Rastafari in Ethiopia David Aarons A dissertation submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy University of Washington 2017 Reading Committee: Shannon Dudley, Chair Giulia Bonacci Katell Morand Christina Sunardi Program Authorized to Offer Degree: Music i @Copyright 2017 David Aarons ii University of Washington Abstract Chanting up Zion: Reggae as Productive Mechanism for Repatriated Rastafari in Ethiopia David Aarons Chair of the Supervisory Committee: Shannon Dudley Ethnomusicology Since the 1960s, Rastafari from Jamaica and other countries have been “returning” to Ethiopia in the belief that it is their Promised Land, Zion. Based on extensive ethnographic research in Ethiopia between 2015 and 2017, this project examines the ways in which repatriated Rastafari use music to transform their Promised Land into a reality amidst various challenges. Since they are denied legal citizenship, Rastafari deploy reggae in creative and strategic ways to gain cultural citizenship and recognition in Ethiopia. This research examines how reggae music operates as a productive mechanism, that is, how human actors use music to produce social and tangible phenomena in the world. Combining theories on music’s productive capabilities with Rastafari ideologies on word-sound, this research further seeks to provide deeper insight into the ways Rastafari effect change through performative arts. I examine how Rastafari mobilize particular discourses that both challenge and reproduce hegemonic systems, creating space for themselves in Ethiopia through music. Rastafari use reggae in strategic ways to insert themselves into the contested national narratives of Ethiopia, and participate in the practice of space-making in Addis Ababa and Shashemene through sound projects. -

WCXR 2004 Songs, 6 Days, 11.93 GB
Page 1 of 58 WCXR 2004 songs, 6 days, 11.93 GB Artist Name Time Album Year AC/DC Hells Bells 5:13 Back In Black 1980 AC/DC Back In Black 4:17 Back In Black 1980 AC/DC You Shook Me All Night Long 3:30 Back In Black 1980 AC/DC Have a Drink on Me 3:59 Back In Black 1980 AC/DC Dirty Deeds Done Dirt Cheap 4:12 Dirty Deeds Done Dirt… 1976 AC/DC Squealer 5:14 Dirty Deeds Done Dirt… 1976 AC/DC Big Balls 2:38 Dirty Deeds Done Dirt… 1976 AC/DC For Those About to Rock (We Salute You) 5:44 For Those About to R… 1981 AC/DC Highway to Hell 3:28 Highway to Hell 1979 AC/DC Girls Got Rhythm 3:24 Highway to Hell 1979 AC/DC Beating Around the Bush 3:56 Highway to Hell 1979 AC/DC Let There Be Rock 6:07 Let There Be Rock 1977 AC/DC Whole Lotta Rosie 5:23 Let There Be Rock 1977 Ace Frehley New York Groove 3:04 Ace Frehley 1978 Aerosmith Make It 3:41 Aerosmith 1973 Aerosmith Somebody 3:46 Aerosmith 1973 Aerosmith Dream On 4:28 Aerosmith 1973 Aerosmith One-Way Street 7:02 Aerosmith 1973 Aerosmith Mama Kin 4:29 Aerosmith 1973 Aerosmith Rattkesnake Shake (live) 10:28 Aerosmith 1971 Aerosmith Critical Mass 4:52 Draw the Line 1977 Aerosmith Draw The Line 3:23 Draw the Line 1977 Aerosmith Milk Cow Blues 4:11 Draw the Line 1977 Aerosmith Livin' on the Edge 6:21 Get a Grip 1993 Aerosmith Same Old Song and Dance 3:54 Get Your Wings 1974 Aerosmith Lord Of The Thighs 4:15 Get Your Wings 1974 Aerosmith Woman of the World 5:50 Get Your Wings 1974 Aerosmith Train Kept a Rollin 5:33 Get Your Wings 1974 Aerosmith Seasons Of Wither 4:57 Get Your Wings 1974 Aerosmith Lightning Strikes 4:27 Rock in a Hard Place 1982 Aerosmith Last Child 3:28 Rocks 1976 Aerosmith Back In The Saddle 4:41 Rocks 1976 WCXR Page 2 of 58 Artist Name Time Album Year Aerosmith Come Together 3:47 Sgt. -

Versions, Dubs and Riddims: Dub and the Transient Dynamics of Jamaican Music
Versions, Dubs and Riddims: Dub and the Transient Dynamics of Jamaican Music Feature Article Thomas Vendryes Ecole Normale Supérieure de Cachan (France) Abstract Dub emerged in Jamaica in the early 1970s, and, for a decade, it became a prolific and intensely innovative dimension of Jamaican popular music. Yet, during the mid- 1980s, while dub flourished at the international level, influencing popular music in general, the genre of dub declined in popularity in Jamaica. How could this musical innovation, so evidently associated with Jamaica, expand and develop internationally while at the same time decline in Jamaica itself? In this paper, I explore the modalities and evolution of Jamaican music production and consumption. Through a description of the Jamaican music industry context, with reference to individual artists’ paths and a summary of Jamaican dub production, I show that even as the Jamaican music milieu was highly favorable to the emergence of dub, dub proliferated as a genre only by developing ties to a diaspora of international audiences and practitioners. Keywords: production studies, Jamaican popular music, history of dub, audio-engineer, riddim, performance mixing Thomas Vendryes is Associate Professor at the Department of Social Sciences of the École Normale Supérieure de Cachan and researcher at the Centre d’Économie de la Sorbonne (France). His research focuses on socio-economic change in developing countries. Dancecult: Journal of Electronic Dance Music Culture 7(2): 5–24 ISSN 1947-5403 ©2015 Dancecult http://dj.dancecult.net http://dx.doi.org/10.12801/1947-5403.2015.07.02.01 6 Dancecult 7(2) “What will happen to dub in 1983?” —Scientist Encounters Pac-man LP (back cover) Dub is Eternal —The 420 DubKraft Anthology Introduction Emerging from the practice of versioning (i.e.