Cell Surface Markers in Acute Lymphoblastic Leukemia* F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Childhood Leukemia
Onconurse.com Fact Sheet Childhood Leukemia The word leukemia literally means “white blood.” fungi. WBCs are produced and stored in the bone mar- Leukemia is the term used to describe cancer of the row and are released when needed by the body. If an blood-forming tissues known as bone marrow. This infection is present, the body produces extra WBCs. spongy material fills the long bones in the body and There are two main types of WBCs: produces blood cells. In leukemia, the bone marrow • Lymphocytes. There are two types that interact to factory creates an overabundance of diseased white prevent infection, fight viruses and fungi, and pro- cells that cannot perform their normal function of fight- vide immunity to disease: ing infection. As the bone marrow becomes packed with diseased white cells, production of red cells (which ° T cells attack infected cells, foreign tissue, carry oxygen and nutrients to body tissues) and and cancer cells. platelets (which help form clots to stop bleeding) slows B cells produce antibodies which destroy and stops. This results in a low red blood cell count ° foreign substances. (anemia) and a low platelet count (thrombocytopenia). • Granulocytes. There are four types that are the first Leukemia is a disease of the blood defense against infection: Blood is a vital liquid which supplies oxygen, food, hor- ° Monocytes are cells that contain enzymes that mones, and other necessary chemicals to all of the kill foreign bacteria. body’s cells. It also removes toxins and other waste products from the cells. Blood helps the lymph system ° Neutrophils are the most numerous WBCs to fight infection and carries the cells necessary for and are important in responding to foreign repairing injuries. -

A Novel Cytogenetic Aberration Found in Stem Cell Leukemia/Lymphoma Syndrome
Letters to the Editor 644 normal PB buffy coat DNA (see example in Figure 1b). These marrow (used for MRD evaluation), the actual Quantitative data show that NSA can be variable, dependent on the type of Range for TCRG targets will often be underestimated. sample (bone marrow or peripheral blood) and the time point We conclude that the ESG-MRD-ALL guidelines for inter- during or after therapy. pretation of RQ-PCR data appropriately take into account the We next evaluated to what extent this variation in NSA variation in NSA. The guidelines for prevention of false-positive affected the RQ-PCR data interpretation, applying the guidelines MRD data perform well, with less than 2% false-positive results. for prevention of false-positive MRD results as well as the However, our data also clearly indicate that positive results guidelines for preventing false-negative MRD results. In Figures outside the Quantitative Range should always be judged with 2a-c, the data interpreted according to the guidelines for the caution, particularly for samples taken after cessation of therapy prevention of false-negative MRD results are shown. IGH targets and analyzed with Ig gene targets. Preferably, one should aim with NSA in normal PB buffy coat DNA resulted in false-positive for RQ-PCR assays without any NSA, since this will improve the MRD data in about 10% of samples obtained during therapy reliability of the data interpretation. (Figure 2a). However, in samples obtained after cessation of therapy (after week 104) false-positivity could be observed in up VHJ van der Velden, JM Wijkhuijs and JJM van Dongen Department of Immunology, Erasmus MC, University Medical to 65% of samples. -

Separation and Functional Studies of the Human Lymphokine-Activated Killer Cell Kevan Roberts, Michael T
[CANCER RESEARCH 47, 4366-4371, August 15, 1987] Separation and Functional Studies of the Human Lymphokine-activated Killer Cell Kevan Roberts, Michael T. Lotze,1 and Steven A. Rosenberg Surgery Branch, National Cancer Institute, NÃŒH,Betnesda,Maryland 20892 ABSTRACT undefined. The purification of cells responsible for LAK activity was desirable to further assess their morphology, phenotype, Cell separation studies were undertaken in an attempt to purify the and target cell binding properties prior to and following IL-2 lymphokine-activated killer (LAK) precursor cell. Null cells, prepared stimulation. by the sequential depletion of monocytes, T- and B-lymphocytes from human peripheral blood mononuclear cells, were found to be potent mediators of LAK activity. Such preparations were Leu-11* but Leu-4" MATERIALS AND METHODS and displayed high levels of natural killer activity. Incubation of these cells with recombinant interleukin 2 (11-2) for periods in excess of 24 h Media. All cell lines were maintained in suspension culture using RPMI 1640 supplemented with 10% FBS, 100 ng/m\ penicillin, 100 induced LAK lysis of fresh tumor targets which were resistant to lysis Mg/ml streptomycin, and 1% glutamine. CM consisted of RPMI 1640 by unstimulated null effectors. In contrast, lymphocytes which formed high affinity rosettes with sheep RBC (I-* lymphocytes) were poor supplemented with 10% human AB serum, antibiotics, and glutamine. Conjugation buffer, at pH 7.3, was prepared by adding 683 mg of NaCl, mediators of both natural killer and LAK activity. Interleukin 2 stimu lated null cells, retained a Leu-ll*, I.t-u-4 phenotype, and expressed 142 mg of MgCl2 6H2O, and 66 mg of CaCl2-6H2O to 450 ml of PBS supplemented with 50 ml of FBS, antibiotics, and 10 HIM4-(2-hydrox- only low levels of receptors for II,-2 and transferrin. -

Genotyping in Patients with Acute Ischemic Stroke
Tuttolomondo et al. Journal of Neuroinflammation (2019) 16:88 https://doi.org/10.1186/s12974-019-1469-5 RESEARCH Open Access HLA and killer cell immunoglobulin-like receptor (KIRs) genotyping in patients with acute ischemic stroke Antonino Tuttolomondo1*† , Domenico Di Raimondo1†, Rosaria Pecoraro6,7, Alessandra Casuccio2, Danilo Di Bona5, Anna Aiello3, Giulia Accardi3, Valentina Arnao4, Giuseppe Clemente1, Vittoriano Della Corte8, Carlo Maida1, Irene Simonetta1, Calogero Caruso3, Rosario Squatrito6, Antonio Pinto1 and on behalf of KIRIIND (KIR Infectious and Inflammatory Diseases) Collaborative Group Abstract Introduction: In humans, a major component of natural killer (NK) and T cell target recognition depends on the surveillance of human leukocyte antigen (HLA) class I molecules by killer immunoglobulin-like receptors (KIRs). Aims: To implement the knowledge about the immunological genetic background of acute ischemic stroke susceptibility in relation to the frequency of the KIR genes and HLA alleles. Methods: Subjects with acute ischemic stroke and subjects without stroke were genotyped for the presence of KIR genes and of the three major KIR ligand groups, HLA-C1, HLA-C2, and HLA-Bw4, both HLA-B and HLA-A loci. Results: Between November 2013 and February 2016, consecutive patients with acute ischemic stroke were recruited. As healthy controls, we enrolled subjects without acute ischemic stroke. Subjects with acute ischemic stroke in comparison with controls showed a higher frequency of 2DL3, 2DL5B, 2DS2, and 2DS4 KIR genes and a lower frequency of HLA-B-Bw4I alleles. Subjects without acute ischemic stroke showed a higher frequency of interaction between KIR 2DS2 and HLAC2. We also observed a higher frequency of 2DL3 and 2 DL4 KIR genes in subjects with atherosclerotic (LAAS) subtype. -

Research Article Segmentation of White Blood Cell from Acute Lymphoblastic Leukemia Images Using Dual-Threshold Method
Hindawi Publishing Corporation Computational and Mathematical Methods in Medicine Volume 2016, Article ID 9514707, 12 pages http://dx.doi.org/10.1155/2016/9514707 Research Article Segmentation of White Blood Cell from Acute Lymphoblastic Leukemia Images Using Dual-Threshold Method Yan Li,1,2 Rui Zhu,1 Lei Mi,1 Yihui Cao,1,2 and Di Yao3 1 State Key Laboratory of Transient Optics and Photonics, Xi’an Institute of Optics and Precision Mechanics of Chinese Academy of Sciences, Xi’an 710119, China 2University of Chinese Academy of Sciences, 52 Sanlihe Road, Beijing 100864, China 3Shenzhen Vivolight Medical Device and Technology Co., Ltd., Shenzhen 518000, China Correspondence should be addressed to Yan Li; [email protected] Received 30 December 2015; Revised 7 April 2016; Accepted 21 April 2016 Academic Editor: Jayaram K. Udupa Copyright © 2016 Yan Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We propose a dual-threshold method based on a strategic combination of RGB and HSV color space for white blood cell (WBC) segmentation. The proposed method consists of three main parts: preprocessing, threshold segmentation, and postprocessing. In the preprocessing part, we get two images for further processing: one contrast-stretched gray image and one H component image from transformed HSV color space. In the threshold segmentationpart,adual-thresholdmethodisproposedforimprovingthe conventional single-threshold approaches and a golden section search method is used for determining the optimal thresholds. For the postprocessing part, mathematical morphology and median filtering are utilized to denoise and remove incomplete WBCs. -

A Hybrid Deep Learning Architecture for Leukemic B-Lymphoblast Classification
A Hybrid Deep Learning Architecture for Leukemic B-lymphoblast Classification Sara Hosseinzadeh Kassani Peyman Hosseinzadeh Kassani Michal J. Wesolowski Department of Computer Science Department of Biomedical Engineering Department of Medical Imaging University of Saskatchewan University of Tulane University of Saskatchewan Saskatoon, Canada New Orleans, USA Saskatoon, Canada [email protected] [email protected] [email protected] Kevin A. Schneider Ralph Deters Department of Computer Science Department of Computer Science University of Saskatchewan University of Saskatchewan Saskatoon, Canada Saskatoon, Canada [email protected] [email protected] Abstract—Automatic detection of leukemic B-lymphoblast can- diagnosed and about 1500 patients are expected to die of ALL, cer in microscopic images is very challenging due to the com- including both children and adults, in the United States. The plicated nature of histopathological structures. To tackle this risk of getting ALL is slightly higher in males than females, issue, an automatic and robust diagnostic system is required for early detection and treatment. In this paper, an automated and higher in whites than African-Americans. However, if deep learning-based method is proposed to distinguish between leukemia is diagnosed in its early stages, it is highly curable immature leukemic blasts and normal cells. The proposed deep and increases the survival rate of the patients. Considering learning based hybrid method, which is enriched by different data the large-scale of histopathology images, assessment of the augmentation techniques, is able to extract high-level features images in a conventional way can be laborious, error-prone from input images. Results demonstrate that the proposed model yields better prediction than individual models for Leukemic B- and hugely time-consuming since some images are highly vari- lymphoblast classification with 96.17% overall accuracy, 95.17% able in morphology which is difficult to analyze. -

Cytotoxic T-Lymphocyte Response to Autologous Human Squamous Cell Cancer of the Lung: Epitope Reconstitution with Peptides Extracted from HLA-Aw68'
[CANCER RESEARCH 54, 2731—2737,May 15, 1994) Cytotoxic T-Lymphocyte Response to Autologous Human Squamous Cell Cancer of the Lung: Epitope Reconstitution with Peptides Extracted from HLA-Aw68' Craig L Slingluff, Jr.,2 Andrea L Cox, John M. Stover, Jr., Marcia M. Moore, Donald F. Hunt, and Victor H. Engeihard Departments ofSurgery (C. L S., J. M. S., M. M. MI, Chemistrj [A. L. C., D. F. H.J, and Mkrobiology [V. H. E.J, University of Virginia, Charlottesville, Virginia 22908 ABSTRACT associated peptides, MHC-unrestricted tumor-specific CTLs have also been described (ii, 12): the peptide backbone of a mucin Cytotoxic T-lymphocytes (Cfls) specific for autologous human squa molecule appears to be the target for some CTLs specific for mom cell cancer of the lung were generated by stimulation of peripheral blood lymphocytes with autologous tumor cells in vitro. The Cl@Lline was carcinomas of the pancreas and of the breast. It is believed that >97% @1J3+,CD8@,CD16andproducedtumornecrosisfactor-a,y-in identification of the peptide epitopes for tumor-specific CTLs will terferon, and granulocyte-macrophage colony-stimulating factor after impact our understanding of the host:tumor relationship and may stimulation with autologous tumor. The CTLs lysed autologous tumor but permit the rational development of novel immunotherapeutic strat failed to recognize autologous or histocompatability leukocyte antigen egies to treat patients with cancer. Although there is evidence of an matched lymphoid cells, K562, or allogeneic tumor cells of several histo immune response to lung cancer, little is known about the target logical types. Antibody-blocking studies suggested that the CTLs re antigens for lung cancer-specific CTLs. -

Table of Contents (PDF)
126 (2) J Immunol 1981; 126:393-810; ; http://www.jimmunol.org/content/126/2.citation This information is current as of October 1, 2021. Downloaded from Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists http://www.jimmunol.org/ • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: by guest on October 1, 2021 http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. THE JOURNAL OF IMMUNOLOGY Volume 126/Number 2 Contents CELLULAR IMMUNOLOGY K. Kudo, A. H. Sehon, and R. J. 403 The Role of Antigen-Presenting Cells in the IgE Antibody Response. I. The Induction Schwenk of High Titer IgE Antibody Responses in IgE High-Responder and Low-Responder Mice by the Administration of Antigen-Pulsed Macrophages in the Absence of Adjuvants D. A. Hubbard, W. Y. Lee, and A. 407 Suppression of the Anti-DNP IgE Response with Tolerogenic Conjugates of DNP H. Sehon with Polyvinyl Alcohol. I. Specific Suppression of the Anti-DNP IgE Response W. Y. Lee and A. H. Sehon 41 4 Suppression of the Anti-DNP IgE Response with Tolerogenic Conjugates of DNP with Polyvinyl Alcohol. -

10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -
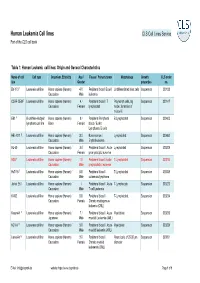
Human Leukemia Cell Lines CLS Cell Lines Service Part of the CLS Cell Bank
Human Leukemia Cell lines CLS Cell Lines Service Part of the CLS cell bank Table 1: Human Leukemia cell lines: Origin and General Characteristics Name of cell Cell type Organism, Ethnicity Age / Tissue / Primary tumor Morphology Growth CLS order line Gender properties no. BV-173 1 Leukemia cell line Homo sapiens (Human) / 47 / Peripheral blood / B cell Undifferentiated blast cells Suspension 300133 Caucasian Male leukemia CCRF-CEM 2 Leukemia cell line Homo sapiens (Human) / 4 / Peripheral blood / T Polymorph cells, big Suspension 300147 Caucasian Female lymphoblast nuclei; formation of microvilli EB1 3 B cell Non-Hodgkin Homo sapiens (Human) / 9 / Peripheral Peripheral B Lymphoblast Suspension 300403 lymphoma cell line Black Female blood / Burkitt Lymphoma, B cells HEL-92.1.7. 4 Leukemia cell line Homo sapiens (Human) / 30 / Bone marrow / Lymphoblast Suspension 300462 Caucasian Male Erythroleukemia HL-60 5 Leukemia cell line Homo sapiens (Human) / 36 / Peripheral blood / Acute Lymphoblast Suspension 300209 Caucasian Female promyelocytic leukemia HSB 6 Leukemia cell line Homo sapiens (Human) / 11 / Peripheral blood / acute T-Lymphoblast Suspension 300214 Caucasian Male lymphoblastic leukemia HuT-78 7 Leukemia cell line Homo sapiens (Human) / 53 / Peripheral blood / T-Lymphoblast Suspension 300338 Caucasian Male cutaneous lymphoma Jurkat E6.1 8 Leukemia cell line Homo sapiens (Human) / / Peripheral blood / Acute T-Lymphocyte Suspension 300223 Caucasian Male T cell Leukemia K-562 9 Leukemia cell line Homo sapiens (Human) / 53 / Peripheral -

Expression of Ligands for Activating Natural Killer Cell Receptors on Cell
Tremblay-McLean et al. BMC Immunology (2019) 20:8 https://doi.org/10.1186/s12865-018-0272-x RESEARCHARTICLE Open Access Expression of ligands for activating natural killer cell receptors on cell lines commonly used to assess natural killer cell function Alexandra Tremblay-McLean1,2, Sita Coenraads1, Zahra Kiani1,2, Franck P. Dupuy1 and Nicole F. Bernard1,2,3,4* Abstract Background: Natural killer cell responses to virally-infected or transformed cells depend on the integration of signals received through inhibitory and activating natural killer cell receptors. Human Leukocyte Antigen null cells are used in vitro to stimulate natural killer cell activation through missing-self mechanisms. On the other hand, CEM.NKr.CCR5 cells are used to stimulate natural killer cells in an antibody dependent manner since they are resistant to direct killing by natural killer cells. Both K562 and 721.221 cell lines lack surface major histocompatibility compatibility complex class Ia ligands for inhibitory natural killer cell receptors. Previous work comparing natural killer cell stimulation by K562 and 721.221 found that they stimulated different frequencies of natural killer cell functional subsets. We hypothesized that natural killer cell function following K562, 721.221 or CEM.NKr.CCR5 stimulation reflected differences in the expression of ligands for activating natural killer cell receptors. Results: K562 expressed a higher intensity of ligands for Natural Killer G2D and the Natural Cytotoxicity Receptors, which are implicated in triggering natural killer cell cytotoxicity. 721.221 cells expressed a greater number of ligands for activating natural killer cell receptors. 721.221 expressed cluster of differentiation 48, 80 and 86 with a higher mean fluorescence intensity than did K562. -

Molecular Regulation of Peripheral B Cells and Their Progeny in Immunity
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Molecular regulation of peripheral B cells and their progeny in immunity Mark R. Boothby,1,2 Emily Hodges,3 and James W. Thomas1,2 1Department of Pathology–Microbiology–Immunology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232, USA; 2Department of Medicine, Rheumatology Division, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA; 3Department of Biochemistry, Vanderbilt Genetics Institute, Nashville, Tennessee 37232, USA Mature B lymphocytes are crucial components of adap- potentials. A vast trove of findings illuminates the tran- tive immunity, a system essential for the evolutionary fit- scriptional regulation and chromatin modifications (for ness of mammals. Adaptive lymphocyte function requires convenience, referred to here as epigenetic) that program an initially naïve cell to proliferate extensively and its developmental progression from common lymphoid pro- progeny to have the capacity to assume a variety of fates. genitors (CLPs) to the establishment of the naïve popu- These include either terminal differentiation (the long- lations of mature T and B cells (e.g., for review, see lived plasma cell) or metastable transcriptional repro- Busslinger 2004; Champhekar et al. 2015). Similarly, the gramming (germinal center and memory B cells). In this process of diversifying subsets of T cells after their activa- review, we focus principally on the regulation of differen- tion has been studied and reviewed intensively (Glimcher tiation and functional diversification of the “B2” subset. and Murphy 2000; Fang and Zhu 2017; Henning et al. An overview is combined with an account of more recent 2018). Mature B lymphocytes also have the potential to advances, including initial work on mechanisms that distribute their progeny among several distinct fates or eliminate DNA methylation and potential links between intermediate states after they have encountered a ligand intracellular metabolites and chromatin editing.