Brazilian Solar Saltworks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Latest Press Release from Consensus Action on Salt and Health
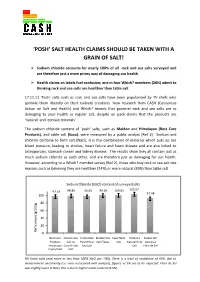
‘POSH’ SALT HEALTH CLAIMS SHOULD BE TAKEN WITH A GRAIN OF SALT! Sodium chloride accounts for nearly 100% of all rock and sea salts surveyed and are therefore just a more pricey way of damaging our health Health claims on labels fuel confusion; one in four Which? members (28%) admit to thinking rock and sea salts are healthier than table salt 17.11.11 ‘Posh’ salts such as rock and sea salts have been popularised by TV chefs who sprinkle them liberally on their culinary creations. New research from CASH (Consensus Action on Salt and Health) and Which? reveals that gourmet rock and sea salts are as damaging to your health as regular salt, despite on pack claims that the products are ‘natural’ and ‘contain minerals’. The sodium chloride content of ‘posh’ salts, such as Maldon and Himalayan (Best Care Products), and table salt (Saxa), were measured by a public analyst [Ref 1]. Sodium and chloride combine to form salt (NaCl), it is this combination of minerals which puts up our blood pressure, leading to strokes, heart failure and heart disease and are also linked to osteoporosis, stomach cancer and kidney disease. The results show they all contain just as much sodium chloride as each other, and are therefore just as damaging for our health. However, according to a Which? member survey [Ref 2], those who buy rock or sea salt cite reasons such as believing they are healthier (24%) or more natural (39%) than table salt. Sodium Chloride (NaCl) content of surveyed salts 98.86 96.65 99.50 100.65 103.57 97.19 91.48 100 80 60 40 20 NaCl contentNaCl (g/100g) 0 Best Care Cornish Sea Halen Mon Maldon Sea Saxa Table Tidman's Zauber der Products Salt Co Pure White Salt Flakes Salt Natural Rock Gewürze Himalayan Cornish Sea Sea Salt Salt Fleur de Sel Crystal Salt Salt NB Some salts total more or less than 100% NaCl per 100g. -

OCCASION This Publication Has Been Made Available to the Public on The
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] MK'Jif iO ipv P[ )| I j Г ¡r irj IP - -T r,HAH î IZSég i Restricted bwirw^l. -

Purified Sea Salt with Magnesium Carbonate
Cargill® Food Processing Salts Purified Sea Saltwith Magnesium Carbonate Product Description Physical Information Purified Sea Salt with Magnesium Carbonate This material is a food grade, granular, white crystalline Purified Sea Salt with Magnesium Carbonate sodium chloride product manufactured under stringent PHYSICAL MIN TARGET MAX process control procedures. Cargill Sea Salts are made from Pacific Ocean sea salt, which is harvested from ponds NaCl (%) 99.7 99.96 100 near the San Francisco Bay. Ca & Mg as Ca (%) 0.003 Sulfate as SO4 (%) 0.01 Product Application Water Insolubles (%) 0.025 0.01 Bulk Density (#cu/ft) 69 74 84 This material is intended for table and cooking use, as well as direct application in foods manufactured by the various Bulk Density (g/l) 1105 1185 1345 food processing industries. This material contains Surface Moisture (%) 0.02 Magnesium Carbonate, which is added to improve caking Magnesium Carbonate (%) 0.5 resistance and flowability. PERCENT PARTICLE SIZE MIN TARGET MAX Product Certifications DISTRIBUTION (SCREENS) Sieve - USS 30 Mesh Retained 0 40 50 Cargill® Sea Salts meet USDA, FDA and Food Chemicals Codex for food use. Sieve - USS 40 Mesh Retained 34 Sieve - USS 50 Mesh Retained 16 Cargill® Sea Salts are certified Kosher for Passover (OU-P) SieveCargill - USS 70 Mesh® RetainedSea Salt 8 by the Orthodox Union. Sieve - Retained on Pan 0 1 10 Made with Sun, Wind and Time Allergen Status Harvesting sea salt from San Francisco Bay today is similar to the salt-making process that has been used for centuries. In accordance with the 2004 USA Food Allergen Labeling and Consumer Protection Act (FALCPA), no allergen declarations are required for this product. -

The Changing Technology of Post Medieval Sea Salt Production in England
1 Heritage, Uses and Representations of the Sea. Centro de Investigação Transdisiplinar Cultura, Espaço e Memoría (CITCEM) Porto, Faculdade de Letras da Universidade do Porto, 20-22 October 2011. The changing technology of post medieval sea salt production in England Jeremy Greenwood Composition of seawater Sea water contains 3.5% evaporites of which salt (sodium chloride) comprises 77.8%. The remainder is known as bittern as it includes the bitter tasting, aperient and deliquescent sulphates of magnesium (Epsom salt) and sodium (Glauber’s salt) as well as about 11% magnesium chloride. 2 Successful commercial salt making depends on the fractional crystallisation of seawater producing the maximum amount of salt without contamination by bittern salts. As seawater is evaporated, very small amounts of calcium carbonate are precipitated followed by some calcium sulphate. This is followed by the crystallisation of sodium chloride but before this is complete, bitter Epsom salt appears; something that needs to be avoided.1 In Continental Europe, evaporation of sea water is achieved solely by the energy of the wind and sun but this is not possible in the English climate so other techniques were developed. 1 http://www.solarsaltharvesters.com/notes.htm SOLAR SALT ENGINEERING 3 Evaporation vessel Briquetage The earliest known English method of coastal saltmaking has been found in the late Bronze Age. This involved boiling seawater in crude clay dishes supported by clay firebars (briquetage) and was widespread in Europe. This technique continued into the Iron Age and into the Roman period with variations inevitably occurring in the industry, although the dating of saltworks is very problematical.2 Detailed interpretation continues to be a matter of dispute. -

LAS VEGAS PRODUCT CATALOG INGREDIENTS Full Page Ad for FINE PASTRY 11”X 8.5”
PRODUCT CATALOG LAS VEGAS chefswarehouse.com BAKING AND PASTRY FROZEN/RTB BREAD ...................12 BEVERAGES, GOAT CHEESE ............................21 CONDIMENTS BAKING JAM ..............................4 PIZZA SHELLS ...............................12 COFFEE AND TEA GOUDA.......................................21 AND JAMS TORTILLAS/WRAPS ......................12 HAVARTI.......................................22 BAKING MIXES ............................4 BAR MIXERS ................................17 CHUTNEY ....................................25 WRAPPERS ..................................12 JACK CHEESE .............................22 BAKING SUPPLIES .......................4 BITTERS .........................................17 GLAZES AND DEMI-GLAZES .......25 BROWNIES ..................................12 MASCARPONE ...........................22 COLORANTS ...............................4 CORDIAL ....................................17 KETCHUP .....................................25 CAKES ASSORTED ......................12 MISCELLANEOUS ........................22 CROISSANTS ...............................4 JUICE ...........................................17 MAYO ..........................................25 TARTS ...........................................13 MOUNTAIN STYLE ........................22 DÉCOR ........................................4 MISCELLANEOUS ........................17 MUSTARD ....................................25 COULIS ........................................13 MOZZARELLA ..............................22 EXTRACTS ....................................6 -

Diversity of Halophilic Archaea in Fermented Foods and Human Intestines and Their Application Han-Seung Lee1,2*
J. Microbiol. Biotechnol. (2013), 23(12), 1645–1653 http://dx.doi.org/10.4014/jmb.1308.08015 Research Article Minireview jmb Diversity of Halophilic Archaea in Fermented Foods and Human Intestines and Their Application Han-Seung Lee1,2* 1Department of Bio-Food Materials, College of Medical and Life Sciences, Silla University, Busan 617-736, Republic of Korea 2Research Center for Extremophiles, Silla University, Busan 617-736, Republic of Korea Received: August 8, 2013 Revised: September 6, 2013 Archaea are prokaryotic organisms distinct from bacteria in the structural and molecular Accepted: September 9, 2013 biological sense, and these microorganisms are known to thrive mostly at extreme environments. In particular, most studies on halophilic archaea have been focused on environmental and ecological researches. However, new species of halophilic archaea are First published online being isolated and identified from high salt-fermented foods consumed by humans, and it has September 10, 2013 been found that various types of halophilic archaea exist in food products by culture- *Corresponding author independent molecular biological methods. In addition, even if the numbers are not quite Phone: +82-51-999-6308; high, DNAs of various halophilic archaea are being detected in human intestines and much Fax: +82-51-999-5458; interest is given to their possible roles. This review aims to summarize the types and E-mail: [email protected] characteristics of halophilic archaea reported to be present in foods and human intestines and pISSN 1017-7825, eISSN 1738-8872 to discuss their application as well. Copyright© 2013 by The Korean Society for Microbiology Keywords: Halophilic archaea, fermented foods, microbiome, human intestine, Halorubrum and Biotechnology Introduction Depending on the optimal salt concentration needed for the growth of strains, halophilic microorganisms can be Archaea refer to prokaryotes that used to be categorized classified as halotolerant (~0.3 M), halophilic (0.2~2.0 M), as archaeabacteria, a type of bacteria, in the past. -

Report on the Composition of Prevalent Salt Varieties
Federal Department of Home Affairs FDHA Federal Food Safety and Veterinary Office FSVO Nutrition Esther Infanger, Max Haldimann, Mai 2016 Report on the composition of prevalent salt varieties 071.1/2013/16500 \ COO.2101.102.7.405668 \ 000.00.61 Contents Summary ................................................................................................................................................. 3 Zusammenfassung .................................................................................................................................. 4 Synthèse .................................................................................................................................................. 5 Sintesi .................................................................................................................................................... 6 1 Introduction ................................................................................................................................. 7 2 Starting point............................................................................................................................... 7 3 Methods ...................................................................................................................................... 8 4 Results ........................................................................................................................................ 9 5 Discussion ............................................................................................................................... -

Epicurean Product Guide 2016 V6.Xlsx
Epicurean Product Listing 2016 800.934.6495 173 Thorn Hill Rd Warrendale, PA 15086 ** For the most up to date listing, please visit our website ** version 6, 9/27/16 EPICUREAN PRODUCT LISTING Condiments.........................................3 Miscellaneous......................................8 Oils & Vinegars................................1210 Syrups.............................................1513 Spices.............................................1715 Dried Mushrooms............................2321 Dried Fruits & Nuts..........................2422 Breads and Crackers.......................2724 Meats & Seafood.............................3027 Pasta Sauces and Noodles.............3330 Desserts..........................................3633 Chocolate........................................4037 Grains & Legumes...........................4340 Cheese, Dairy, & Eggs....................4542 Bar & Bakery.......................................47 Baking & Pastry...................................50 Appetizers...........................................61 CONDIMENTS Prod # Description Packaging UoM Special Order 06206 BASE BEEF NO MSG 4/5 LB CS 06207 BASE BEEF NO MSG 5 LB EA 06176 BASE BEEF NO MSG MINORS 12/1 LB CS X 06179 BASE CHICKEN NO MSG 1 LB EA 06201 BASE CHICKEN NO MSG 5 LB EA 06178 BASE CHICKEN NO MSG MINORS 12/1 LB CS 06200 BASE CHICKEN NO MSG MINORS 4/5 LB CS 06180 BASE CLAM NO MSG MINORS 6/1 LB CS 06181 BASE CLAM NO MSG MINORS 1# EA 06198 BASE CRAB NO MSG MINORS 6/1 LB CS 06199 BASE CRAB NO MSG MINORS 1# EA 06187 BASE ESPAGNOLE SAUCE -

Common Salt Without Additives Safety Data Sheet According to Federal Register / Vol
Common Salt without Additives Safety Data Sheet According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations And According To The Hazardous Products Regulation (February 11, 2015). Date of Issue: 05/27/2021 Version: 1.0 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Substance Product Name: Common Salt without Additives Synonyms: All purpose natural sea salt; All purpose Purex salt; Bulk Culinox 999 NC, Bulk extra coarse solar undried NC; Bulk KD Industrial Salt NC; Bulk Purex Salt NC; Bulk Rock salt NOC 17F NC; Bulk Rock WC extra coarse southern NC; Bulk Rock white crystal coarse southern NC; Bulk solar coarse salt undried NC, Bulk solar industrial crude salt NC; Bulk solar salt; Bulk solar WC extra coarse salt NC; Bulk solar white crystal coarse salt NC; Bulk solar white crystal medium salt NC; Bunny spool (plain salt), California pure coarse sea salt; California pure medium sea salt; California pure fine sea salt; Canning & Pickling Salt; Coarse sea salt (F114100000x); Commercial grade water softening pellets; Culinox 999 chemical grade salt; Culinox 999 fine salt; Culinox 999 food grade salt; Evaporated granulated salt; Evaporated salt pellets; Extra coarse sea salt; Extra fine 50 sea salt; Extra fine 70 sea salt; Feed mixing salt; Fine solar salt (w/o YPS); Hi-Purity super soft salt extra coarse crystals; Himalayan pink salt; H.G. blending salt; Hay & Stock salt, F&R; Industrial crude solar salt; ISCO crystals, bulk; ISCO medium, bulk; ISCO water conditioning, bulk; KD crude solar -

KANSAS July 5, 2017 9:00A.M
CITY OF HUTCHINSON AGENDA CITY COUNCIL MEETING COUNCIL CHAMBERS_;_ HUTCHINSON, KANSAS July 5, 2017 9:00a.m. 1. ROLL CALL Piros de Carvalho __ Soldner __ Inskeep __ Dechant__ Daveline __ 2. PLEDGE OF ALLEGIANCE TO THE FLAG 3. PRAYER 4. PROCLAMATIONS a. Parks and Recreation Month 5. PETITIONS, REMONSTRANCES, AND COMMUN/CA tlONS a. Oral communications from the audience. (Please limit your remarks to five (5) minutes and to items NOT on the agenda.) 6. CONSENT AGENDA a. Approval of Minutes of June 20, 2017 City Council meeting. b. Approval of License Agreement with C&M Investment LLC for private sewer th service in Maple Street right-of-way at 229 East 10 . c. Approval of appointments to the Hutchinson Housing Commission of the following: Nathan DeBerry, 2 West 21st, for a first three-year term beginning 6/29/2017 to 6/29/2020. Ryan Patton, 3504 Rockwood Drive, for a first three-year term beginning 6/29/2017 to 6/29/2020. d. Approval of appropriation ordinance in the amount of $2,040,307.12. Action - Motion to approve the Consent Agenda; and authorize the Mayor to sign. Motion-----~----- Second __________ Piros de Carvalho __ Soldner __ Inskeep __ Dechant__ Daveline __ City Council Agenda July 5, 2017 Page 2 7. ORDINANCES AND RESOLUTIONS a. Consider Ordinance pledging future use of quarter cent retailers' sales tax revenue. Action - Motion to approve an Ordinance pledging the future use of the revenue which may be received by the City of Hutchinson from the one-fourth of one percent retailers' sales tax levied in the City of Hutchinson, Kansas; and authorize the Mayor to sign. -

Solar Salt Technology in Ghana – a Case Study of Small Scale Salt Winning Process
Ghana J. Sci. 46 (2006), 99-109 SOLAR SALT TECHNOLOGY IN GHANA – A CASE STUDY OF SMALL SCALE SALT WINNING PROCESS B. MENSAH AND R. BAYITSE CSIR – Institute of Industrial Research, P. O. Box M.32, Accra, Ghana Abstract Résumé Generally, unrefined salt from local small scale salt MENSAH B. & BAYITSE R.: Technologie solaire de sel au producers does not meet the standard specifications Ghana - Une étude de cas du procé dé de salinage of the Ghana Standard Board. In this study, the peu important. En général le sel non raffiné de saliniers traditional small scale salt production process was locaux peu importants ne correspond pas aux studied. Brine and salt samples from the various spécifications normales du Conseil Ghanéen de stages of the traditional process were analysed for Normalisation. Dans cette étude le procédé calcium, sulphate, magnesium and chloride contents traditionnel relatid à la production de sel peu impor- in order to propose a method to improve the quality tant, était étudié. L’eau salée et les échantillons de sel of output. The brine samples contained low levels of prélevés de différents stades du procédé traditionnel calcium (ranging from 0.14% to 0.01% ), and high étaient analysés pour les teneurs en calcium, en sul- levels of magnesium (ranging from 1.04% to 4.49%). fate, en magnésium et en chlorure afin de proposer These two elements constitute the two main une méthode pour améliorer la qualité de la produc- elemental impurities found in common salt. Samples tion. Les échantillons contenaient de faibles niveaux of salt produced also contain high levels of de calcium et de niveaux élevés de magnésium, les magnesium, averaging 0.98 per cent, above the deux impuretés élémentaires principales qui se trouvent recommended maximum level of 0.1 per cent by the dans le sel ordinaire. -

Saltworks by Jennifer Stone Gaines and John York
From Spritsail: A Journal of the History of Falmouth and Vicinity, Vol. 21, No. 1. Winter, 2007. Woods Hole Historical Collection, Woods Hole, MA Saltworks by Jennifer Stone Gaines and John York In Colonial days, a large quantity of salt was abso England fisheries until the end of the 1600s when lutely essential to life in New England, both for food the development of Mediterranean style salt works preservation and for income. One of the best ways in the British Caribbean islands made high quality to preserve fish and meat was to salt it. To preserve salt available to all the British colonies. Salt became cod, the fish were gutted, beheaded, and put into a common return cargo for vessels carrying New wooden barrels, layered with salt - almost as much England exports, primarily timber and salt fish, to salt as fish. The fish sat in the barrels for a week to the Caribbean colonies. ten days while the salt pulled moisture out of the fish. The barrels were then ope:nea , tne orine aumpea out, This arrangement worked well until the Ameri and then the fish put ba ck into the barrels, layered can colonies began with fresh salt. After to chafe under Brit several more days, the ish restrictions. The brine was dumped British government out and the fish were imposed not only the spread on racks to infamous tax on tea, dry in the sun. When but also a salt tax. thoroughly dry, the Since salt was criti fish were put into cal to the livelihood clean dry barrels, of the Massachusetts ready for export.