Cultivars of Chestnuts Updated January 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer
Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer For 60 years the work of Frank N. Meyer has 2,500 pages of his letters tell of his journeys remained a neglected segment of America’s and the plants he collected, and the USDA heritage. Now, as people are becoming con- Inventory of Seeds and Plants Imported con- cerned about feeding the world’s growing tains descriptions of his introductions. population and about the loss of genetic di- Until recently little was known about the versity of crops, Meyer’s accomplishments first 25 years of Meyer’s life, when he lived have a special relevance. Entering China in in Amsterdam and was called Frans Meijer. 1905, near the dawn of the single era when Dutch sources reveal that he was bom into a explorers could travel freely there, he be- loving family in 1875. Frans was a quiet boy, came the first plant hunter to represent a who enjoyed taking long walks, reading government and to search primarily for eco- about distant lands, and working in his fami- nomically useful plants rather than orna- ly’s small garden. By the time he had mentals. No one before him had spent 10 fimshed elementary school, he knew that ’ years crossing the mountains, deserts, farms, wanted to be a world traveler who studied he ’,if and forests of Asia in search of fruits, nuts, plants; however, his parents could not afford vegetables, grains, and fodder crops; no one to give him further education. When he was has done so since. 14 years old, he found work as a gardener’s During four plant-hunting expeditions to helper at the Amsterdam Botanical Garden. -

Castanea Mollissima Blume): the Roots of Nut Tree Domestication
ORIGINAL RESEARCH published: 25 June 2018 doi: 10.3389/fpls.2018.00810 Signatures of Selection in the Genomes of Chinese Chestnut (Castanea mollissima Blume): The Roots of Nut Tree Domestication Nicholas R. LaBonte 1*, Peng Zhao 2 and Keith Woeste 3 1 Department of Crop Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States, 2 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China, 3 Hardwood Tree Improvement and Regeneration Center, Northern Research Station, USDA Forest Service, West Lafayette, IN, United States Chestnuts (Castanea) are major nut crops in East Asia and southern Europe, and are unique among temperate nut crops in that the harvested seeds are starchy rather than oily. Chestnut species have been cultivated for three millennia or more in China, so it is likely that artificial selection has affected the genome of orchard-grown chestnuts. The genetics of Chinese chestnut (Castanea mollissima Blume) domestication are also of Edited by: interest to breeders of hybrid American chestnut, especially if the low-growing, branching S. Hong Lee, habit of Chinese chestnut, an impediment to American chestnut restoration, is partly University of South Australia, Australia the result of artificial selection. We resequenced genomes of wild and orchard-derived Reviewed by: Guo-Bo Chen, Chinese chestnuts and identified selective sweeps based on pooled whole-genome SNP Zhejiang Provincial People’s Hospital, datasets. We present candidate gene loci for chestnut domestication and discuss the China potential phenotypic effects of candidate loci, some of which may be useful genes for Chaeyoung Lee, Soongsil University, South Korea chestnut improvement in Asia and North America. -

Chestnut Growers' Guide to Site Selection and Environmental Stress
This idyllic orchard has benefited from good soil and irrigation. Photo by Tom Saielli Chestnut Growers’ Guide to Site Selection and Environmental Stress By Elsa Youngsteadt American chestnuts are tough, efficient trees that can reward their growers with several feet of growth per year. They’ll survive and even thrive under a range of conditions, but there are a few deal breakers that guarantee sickly, slow-growing trees. This guide, intended for backyard and small-orchard growers, will help you avoid these fatal mistakes and choose planting sites that will support strong, healthy trees. You’ll know you’ve done well when your chestnuts are still thriving a few years after planting. By then, they’ll be strong enough to withstand many stresses, from drought to a caterpillar outbreak, with much less human help. Soil Soil type is the absolute, number-one consideration when deciding where—or whether—to plant American chestnuts. These trees demand well-drained, acidic soil with a sandy to loamy texture. Permanently wet, basic, or clay soils are out of the question. So spend some time getting to know your dirt before launching a chestnut project. Dig it up, roll it between your fingers, and send in a sample for a soil test. Free tests are available through most state extension programs, and anyone can send a sample to the Penn State Agricultural Analytical Services Lab (which TACF uses) for a small fee. More information can be found at http://agsci.psu.edu/aasl/soil-testing. There are several key factors to look for. The two-foot-long taproot on this four- Acidity year-old root system could not have The ideal pH for American chestnut is 5.5, with an acceptable range developed in shallow soils, suggesting from about 4.5 to 6.5. -

5 Fagaceae Trees
CHAPTER 5 5 Fagaceae Trees Antoine Kremerl, Manuela Casasoli2,Teresa ~arreneche~,Catherine Bod6n2s1, Paul Sisco4,Thomas ~ubisiak~,Marta Scalfi6, Stefano Leonardi6,Erica ~akker~,Joukje ~uiteveld', Jeanne ~omero-Seversong, Kathiravetpillai Arumuganathanlo, Jeremy ~eror~',Caroline scotti-~aintagne", Guy Roussell, Maria Evangelista Bertocchil, Christian kxerl2,Ilga porth13, Fred ~ebard'~,Catherine clark15, John carlson16, Christophe Plomionl, Hans-Peter Koelewijn8, and Fiorella villani17 UMR Biodiversiti Genes & Communautis, INRA, 69 Route d'Arcachon, 33612 Cestas, France, e-mail: [email protected] Dipartimento di Biologia Vegetale, Universita "La Sapienza", Piazza A. Moro 5,00185 Rome, Italy Unite de Recherche sur les Especes Fruitikres et la Vigne, INRA, 71 Avenue Edouard Bourlaux, 33883 Villenave d'Ornon, France The American Chestnut Foundation, One Oak Plaza, Suite 308 Asheville, NC 28801, USA Southern Institute of Forest Genetics, USDA-Forest Service, 23332 Highway 67, Saucier, MS 39574-9344, USA Dipartimento di Scienze Ambientali, Universitk di Parma, Parco Area delle Scienze 1lIA, 43100 Parma, Italy Department of Ecology and Evolution, University of Chicago, 5801 South Ellis Avenue, Chicago, IL 60637, USA Alterra Wageningen UR, Centre for Ecosystem Studies, P.O. Box 47,6700 AA Wageningen, The Netherlands Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556, USA lo Flow Cytometry and Imaging Core Laboratory, Benaroya Research Institute at Virginia Mason, 1201 Ninth Avenue, Seattle, WA 98101, -
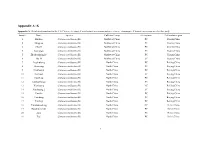
Appendix A~K
Appendix A~K Appendix A. Detailed information for the 146 Chinese chestnut (C.mollissima) accessions and nine chinese chinquapin (C.henryi) accessions used in this study Sample Name Species Cultivars Group Germplasm Cultivation region 1 Huijian Castanea mollissima Bl. Northwest China PC Shanxi,China 2 Mingjian Castanea mollissima Bl. Northwest China PC Shanxi,China 3 Chunli Castanea mollissima Bl. Northwest China PC Shanxi,China 4 Zuohongli Castanea mollissima Bl. Northwest China PC Shanxi,China 5 Zhenbashuangjie Castanea mollissima Bl. Northwest China PC Shanxi,China 6 zha 18 Castanea mollissima Bl. Northwest China LC Shanxi,China 7 Jingshuhong Castanea mollissima Bl. North China PC Beijing,China 8 Huaixiang Castanea mollissima Bl. North China PC Beijing,China 9 Huaihuang Castanea mollissima Bl. North China PC Beijing,China 10 Huzhaoli Castanea mollissima Bl. North China LC Beijing,China 11 Huaifeng Castanea mollissima Bl. North China PC Beijing,China 12 Yanshanhongli Castanea mollissima Bl. North China PC Beijing,China 13 Xiazhuang Castanea mollissima Bl. North China LC Beijing,China 14 Xinzhuang 2 Castanea mollissima Bl. North China LC Beijing,China 15 Huaijiu Castanea mollissima Bl. North China PC Beijing,China 16 Yanchang Castanea mollissima Bl. North China PC Beijing,China 17 Yanfeng Castanea mollissima Bl. North China PC Beijing,China 18 Yanshanzaofeng Castanea mollissima Bl. North China NC Hebei,China 19 Donglimingzhu Castanea mollissima Bl. North China PC Hebei,China 20 Zipo Castanea mollissima Bl. North China PC Hebei,China 21 Tasi Castanea mollissima Bl. North China PC Hebei,China 1 22 Zundali Castanea mollissima Bl. North China PC Hebei,China 23 Duanzhiyabian Castanea mollissima Bl. -

Mastroberardino Radici Taurasi Docg 2016
MASTROBERARDINO RADICI TAURASI DOCG 2016 BACKGROUND The Mastroberardino family, leaders in Italian viticulture, are largely responsible for the revival and elevation of Irpinian and Campanian winemaking post WWII. Their efforts ignited a resurgence in quality red and white wine production in all of Southern Italy. Working primarily with Campania’s ancient, native varietals, Fiano, Greco, and Aglianico, Mastroberardino has resuscitated would-be extinct grapes into world class varieties. Radici Taurasi is Mastroberardino’s flagship wine. Although first released in 1928, the wine became known as Radici, which translates as “roots,” in 1986. This classic red is the result of painstaking research into Aglianico clonal selection, rootstocks, and site selection of this region’s unique volcanic terroir and topography. A brilliant example of the powerful structure and elegance of this noble grape. APPELLATION Taurasi DOCG, Italy VARIETAL COMPOSITION 100% Aglianico TERROIR & VINTAGE NOTES Grapes are sourced from the two estate vineyards in Montemarano and Mirabella Eclano. Montemarano is a historic site in Taurasi VINOUS production, in the southernmost part of the DOCG area. It has a South- 95 May 2020 East exposure and clay soil. The vineyard sits at 500-650m a.s.l. and POINTS has an average vine age of 20 years. The vines are cordon spur- 93 PTS WINE & SPIRITS 12/20 pruned with espalier training and plated at a density of about 4,000 91 PTS WINE SPECTATOR 10/20 vines/hectare (or 1,600 vines/acre). Yields average 5,000 kg/ha (or 4,460 lbs/acre) and 1.3 kg/vine (or 2.87 lbs/vine). -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

CCOF ~ 30Th Anniversary
the newsletter o f California Certified Organic Farmers Volume XIX, Number 4 Creating a Living Standard for Healthy Food Winter 2002–2003 CCOF’s Anniversary! th page 24 30 ORGANIC LIVESTOCK page 2 $3.50 BROCCOLI: CROWN JEWEL EVALUATING COMPOST ORGANIC RAW MILK LAWN CARE CHEMICALS page 8 page 16 page 38 page 49 FIRST WORD HE UIET RUTH Farmers that have been exposed to 2,4,5,- ble. Once the true costs of toxic chemical T Q T T and 2,4-D have two- to eightfold agriculture are determined and placed on ABOUT CONVENTIONAL increases in non-Hodgkin’s lymphoma products, then organic agriculture will over farmers who have not been exposed dominate in the marketplace. Before a true AGRICULTURE to those two chemicals. Herbicides such as accounting system is adopted, our own chlorophenoxy have been government must regain By Brian Leahy CCOF President linked to increased rates of a value system that puts immune cancers in migrant Toxic chemicals are human life, the environ- SPENT THIS PAST and seasonal farm workers, ment, and culture above FOURTH OF JULY at a as well as county extension attacking our bodies and the desires of the agro- friend’s family farm agents. Childhood cancers chemical interest group I have also been shown to be that has dominated in Kentucky. My friend’s our families’ bodies, our soil father confided in me that he believes the linked with the toxic chem- USDA and the land high cancer rate in his family is a result of ical tools that family farms and the life within and grant colleges for the the tools he used to produce his crops. -

Download Colour Chart
DIRECT DYE * AMMONIA FREE * PEROXIDE FREE DIRECTIONS: CUSTOMISED COLOUR MAINTENANCE APPLICATION MIXING TIMING refer to menu for depending on hair condition suggested formulas and desired colour intensity colour maintenance add 80g fab pro 3 minutes conditioner formula (direct dye match and maintain colour or direct dye and in-between salon visits. conditioner) to 200ml conditioner base. shake well. MATCH IT * MIX IT * TAKE IT AWAY DIRECTIONS: COLOUR SERVICE 1. identify the level, tone and length of hair. 2. select the appropriate colour formula from the mixologist menu, ensuring the formula works with the lightest level of hair. 3. measure and mix your formula. 4. prepare hair by shampooing, towel-dry hair evenly, then detangle and comb through. APPLICATION MIXING TIMING refer to menu for depending on hair condition suggested formulas and desired colour intensity colour refresh mix fab pro direct 5 – 15 minutes refresh existing hair colour dyes together. on mid-lengths and ends in-between all colour services (permanent, demi and semi- permanent colour). colour fill* mix fab pro direct 5 – 15 minutes darken lighter hair quickly, dyes together. without damage. *for filling formulas, see fab pro fill chart or visit evohair.com. colour tone* / pastels mix fab pro direct 5 – 15 minutes ideal for colour toning and dyes together or with can be diluted to create pastel shades. conditioner base. *for extremely porous hair, you may need to dilute fab pro direct dye using conditioner base. DIRECT DYES LEVEL 5 LEVEL 7 LEVEL 10 colour results are determined by the level, tone and formula you use. in order to accurately predict a colour result, you need to understand how the existing level and tone will contribute to the result; the lighter the level, the more intense the result. -

The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea Ozarkensis" (2017)
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2017 The ffecE t of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis Colton Zirkle University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/etd Part of the Botany Commons, Entomology Commons, and the Plant Biology Commons Recommended Citation Zirkle, Colton, "The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis" (2017). Theses and Dissertations. 1996. http://scholarworks.uark.edu/etd/1996 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected], [email protected]. The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology by Colton Zirkle Missouri State University Bachelor of Science in Biology, 2014 May 2017 University of Arkansas This thesis is approved for recommendation to the Graduate Council. ____________________________________ Dr. Ashley Dowling Thesis Director ____________________________________ ______________________________________ Dr. Frederick Paillet Dr. Neelendra Joshi Committee Member Committee Member Abstract Ozark chinquapin (Castanea ozarkensis), once found throughout the Interior Highlands of the United States, has been decimated across much of its range due to accidental introduction of chestnut blight, Cryphonectria parasitica. Efforts have been made to conserve and restore C. ozarkensis, but success requires thorough knowledge of the reproductive biology of the species. Other Castanea species are reported to have characteristics of both wind and insect pollination, but pollination strategies of Ozark chinquapin are unknown. -

An Evolutionary Perspective on Human Cross-Sensitivity to Tree Nut and Seed Allergens," Aliso: a Journal of Systematic and Evolutionary Botany: Vol
Aliso: A Journal of Systematic and Evolutionary Botany Volume 33 | Issue 2 Article 3 2015 An Evolutionary Perspective on Human Cross- sensitivity to Tree Nut and Seed Allergens Amanda E. Fisher Rancho Santa Ana Botanic Garden, Claremont, California, [email protected] Annalise M. Nawrocki Pomona College, Claremont, California, [email protected] Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, Evolution Commons, and the Nutrition Commons Recommended Citation Fisher, Amanda E. and Nawrocki, Annalise M. (2015) "An Evolutionary Perspective on Human Cross-sensitivity to Tree Nut and Seed Allergens," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 33: Iss. 2, Article 3. Available at: http://scholarship.claremont.edu/aliso/vol33/iss2/3 Aliso, 33(2), pp. 91–110 ISSN 0065-6275 (print), 2327-2929 (online) AN EVOLUTIONARY PERSPECTIVE ON HUMAN CROSS-SENSITIVITY TO TREE NUT AND SEED ALLERGENS AMANDA E. FISHER1-3 AND ANNALISE M. NAWROCKI2 1Rancho Santa Ana Botanic Garden and Claremont Graduate University, 1500 North College Avenue, Claremont, California 91711 (Current affiliation: Department of Biological Sciences, California State University, Long Beach, 1250 Bellflower Boulevard, Long Beach, California 90840); 2Pomona College, 333 North College Way, Claremont, California 91711 (Current affiliation: Amgen Inc., [email protected]) 3Corresponding author ([email protected]) ABSTRACT Tree nut allergies are some of the most common and serious allergies in the United States. Patients who are sensitive to nuts or to seeds commonly called nuts are advised to avoid consuming a variety of different species, even though these may be distantly related in terms of their evolutionary history.