Pericardial Effects on Diastolic Ventricular Interaction During Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -
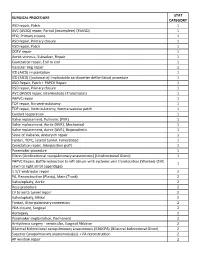
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon
r Me ula dic sc in a e V & f o S l u a Journal of Vascular r Nath et al., J Vasc Med Surg 2014, 2:2 g n r e u r y o DOI: 10.4172/2329-6925.1000135 J ISSN: 2329-6925 Medicine & Surgery Short Communication Open Access Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon Mridu Paban Nath1*, Malavika Barman2 and Rajib Kr Bhattacharrya3 1Assistant Professor, Department of Anesthesiology & Critical Care, Gauhati Medical College Hospital, Assam, India 2Assistant Professor, Department of Biochemistry, Tezpur Medical College Hospital, Assam, India 3Professor & Head, Department of Anesthesiology & Critical Care, FAA Medical College Hospital, Assam, India A 28 year old boy was referred from a private hospital for evaluation long periods of myocardial compression contributing to remodelling of constrictive pericarditis. He was diagnosed for the same about 4 of the ventricles and to greater involvement of the myocardium in years back with history of worsening shortness of breath and fatigue. patients who have undergone long periods of symptomatic pericardial At the time of presentation, patient required supplemental Oxygen constriction, as in our patient with a history of 4 years of symptoms. and was New York Heart Association Class-IV heart failure. Physical MacCaughan et al. [4] have described haemodynamic abnormalities examination revealed distension of jugular veins with significant after pericardiectomy in the largest series available (231 patients). The ascites & hepatomegaly. Bilateral pedal edema was absent; however investigators noted a 28% incidence of LCOS postoperatively in their patient was on long term therapy with loop diuretics. About 1 litre of patients, with many of the perioperative deaths occurring in this low abdominal paracentesis was done to relieve tense ascites. -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

Prognostic Predictors in Pericardiectomy for Chronic Constrictive Pericarditis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Acquired Cardiovascular Disease Kang et al Prognostic predictors in pericardiectomy for chronic constrictive pericarditis Se Hun Kang, MD,a Jong-Min Song, MD, PhD,a Minsoo Kim, MD,a Suk Jung Choo, MD, PhD,b Cheol Hyun Chung, MD, PhD,b Duk-Hyun Kang, MD, PhD,a and Jae-Kwan Song, MD, PhDa Objective: Prognosis after pericardiectomy remains to be clearly elucidated, especially in Asian countries, ACD where the causes of constrictive pericarditis differ from those in Western countries. We aimed to investigate the preoperative prognostic factors and clinical outcomes after pericardiectomy in patients with chronic constrictive pericarditis. Methods: Preoperative clinical and imaging characteristics were evaluated in 85 consecutive patients with chronic constrictive pericarditis without other valvular or ischemic heart diseases who underwent pericardiec- tomy. Causes were idiopathic in 49 patients (57.6%) and tuberculous in 36 patients (42.4%). All-cause death was observed for a median of 38.5 months. Results: Of 85 patients, 15 (17.6%) died during follow-up. These 15 patients who died during follow-up had higher aspartate aminotransferase, smaller left ventricular end-systolic dimension index, and higher early dia- stolic mitral inflow velocity before pericardiectomy than the 70 patients who survived. Multivariate Cox propor- tional analysis showed that diabetes mellitus (hazard ratio, 4.610; P ¼ .024) and high early diastolic mitral inflow velocity (hazard ratio, 1.050/cm/s; P ¼ .002) before pericardiectomy were independent predictors of mortality after pericardiectomy. The preoperative cutoff value for early diastolic mitral inflow velocity in pre- dicting mortality after pericardiectomy was 71 cm/s (sensitivity of 84.6% and specificity of 52.2%), and there was a significant difference in survival between groups divided by this cutoff value of early diastolic mitral inflow velocity (P ¼ .029). -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

1 United States of America Department of Health And
1 UNITED STATES OF AMERICA DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION + + + CENTER FOR DEVICES AND RADIOLOGICAL HEALTH MEDICAL DEVICES ADVISORY COMMITTEE + + + CIRCULATORY SYSTEM DEVICES PANEL + + + April 6, 2021 9:00 a.m. Via Microsoft Teams Videoconference PANEL MEMBERS: RICHARD A. LANGE, M.D., M.B.A. Panel Chair GEORGE W. VETROVEC, M.D., MACC, MSCAI Voting Member JAMES C. BLANKENSHIP, M.D. Voting Member JASON T. CONNOR, Ph.D. Voting Member RALPH G. BRINDIS, M.D., M.P.H., MACC, FSCAI Voting Member KEITH B. ALLEN, M.D. Voting Member MURRAY KWON, M.D. Temporary Voting Member PRAMOD BONDE, M.D. Temporary Voting Member COLLEEN M. GALLAGHER, Ph.D. Temporary Voting Member ALFRED H. STAMMERS, MSA, CPBMT, CCP Temporary Voting Member DAVID YUH, M.D. Temporary Voting Member ROBERT W. YEH, M.D., M.Sc., M.B.A. Temporary Voting Member CRAIG SELZMAN, M.D. Temporary Voting Member MARC R. KATZ, M.D. Temporary Voting Member JOAQUIN E. CIGARROA, M.D. Temporary Voting Member JOHN HIRSHFELD, M.D. Temporary Voting Member ALLEN BURKE, M.D. Temporary Voting Member MARK NUSZKOWSKI, M.D. Temporary Voting Member Free State Reporting, Inc. 1378 Cape Saint Claire Road Annapolis, MD 21409 (410) 974-0947 2 CHRISTOPHER O’ CONNOR, M.D., MACC Temporary Voting Member JEFFREY BORER, M.D. Temporary Voting Member MARC MOON, M.D. Temporary Voting Member DEBRA DUNN Patient Representative GARY JARVIS Industry Representative ADEN ASEFA, M.P.H. Designated Federal Officer Free State Reporting, Inc. 1378 Cape Saint Claire Road Annapolis, MD 21409 (410) 974-0947 3 FDA REPRESENTATIVES: BRAM ZUCKERMAN, M.D. -

Aortic Valve Replacement in Rheumatoid Aortic Incompetence
Thorax: first published as 10.1136/thx.33.5.612 on 1 October 1978. Downloaded from Thorax, 1978, 33, 612-615 Aortic valve replacement in rheumatoid aortic incompetence A B DEVLIN, P GOLDSTRAW, AND P K CAVES From the University Department of Cardiac Surgery, Royal Infirmary, Glasgow G4 OSF, UK Devlin, A B, Goldstraw, P, and Caves, P K (1978). Thorax, 33, 612-615. Aortic valve replacement in rheumatoid aortic incompetence. Rheumatoid aortic valve disease is uncommon, and there are few reports of valve replacement in this condition. Aortic valve replacement and partial pericardiectomy was performed in a patient with acute rheumatoid aortitis and aortic incompetence. Previous reports suggest that any patient with rheumatoid arthritis who develops cardiac symptoms should be carefully assessed for surgically treatable involvement of the pericardium or heart valves. The commonest symptomatic cardiac valvar were collapsing in character, and his blood pres- lesion in patients with rheumatoid arthritis is sure was 170/35 mmHg. Auscultation showed aortic incompetence, but aortic stenosis (Lassiter loud aortic systolic and diastolic murmurs. He had copyright. and Tassy, 1965) and mitral regurgitation (Car- "pistol-shot" femoral pulses. There was rheuma- penter et al, 1967) have been reported. Rupture toid arthritis of his elbow, wrist, knee, and ankle of the sinus of Valsalva with complete heart joints. block has also been described (Howell et al, 1972). His plain chest radiograph showed cardiomegaly This clinical experience contrasts with post- with pulmonary oedema. His electrocardiogram http://thorax.bmj.com/ mortem studies that show the similarity of rheu- showed left ventricular hypertrophy with strain, matoid and rheumatic cardiac disease with mitral, the cardiac rhythm varying between sinus tachy- aortic, tricuspid, and pulmonary valvar involve- cardia and atrial fibrillation. -

Aortic Valve Replacement Via a Right Parasternal Approach in a Patient
Nakayama and Asano Surgical Case Reports (2019) 5:39 https://doi.org/10.1186/s40792-019-0598-5 CASE REPORT Open Access Aortic valve replacement via a right parasternal approach in a patient with a history of coronary artery bypass surgery and pericardiectomy: a case report Takuya Nakayama* and Miki Asano Abstract Background: The number of patients who require aortic valve replacement after coronary artery bypass grafting continues to increase. Re-operative cardiovascular surgery after coronary artery bypass grafting has various risk factors related to median re-sternotomy. It is particularly essential to avoid damage to the living graft. We successfully performed aortic valve replacement via right parasternal thoracotomy in a patient who had undergone coronary artery bypass grafting. Case presentation: An 80-year-old man who had undergone coronary artery bypass grafting was referred to our hospital for syncope caused by severe aortic valve stenosis. He also had a history of pericardiotomy for constrictive pericarditis. His left internal thoracic artery bypass graft was patent. Aortic valve replacement was performed through a small right parasternal thoracotomy during cardiac arrest following cardiopulmonary bypass under moderate hypothermia and hyperkalemia by intermittent selective antegrade cardioplegia. His postoperative course was uneventful. Conclusion: Aortic valve replacement via right parasternal thoracotomy with moderate hypothermia and hyperkalemia was safe and effective for avoidance of re-sternotomy-related complications. Keywords: Minimally invasive, Aortic valve replacement, Coronary artery bypass surgery, Right parasternal approach Background permanent pacemaker implantation and paravalvular The number of patients who require aortic valve replace- leakage are higher in patients undergoing TAVR [4, 5]. ment for aortic valve stenosis (AS) long after coronary Therefore, AVR after CABG might have fewer risks if artery bypass grafting (CABG) continues to increase [1]. -

Constrictive Pericarditis
Central Journal of Cardiology & Clinical Research Research Article *Corresponding author Prof. Sushil K Singh, Professor and Head, Department of Cardiovascular and Thoracic surgery, King George’s Constrictive Pericarditis - A Medical University, Lucknow, 226003, India, Tel and Fax: 91-522-2258830; Email: [email protected] Submitted: 30 July 2020 Long Term Surgical Experience Accepted: 14 August 2020 Published: 18 August 2020 Sarvesh Kumar, Vivek Tewarson, Mohammad Zeeshan Hakim, Copyright Shobhit Kumar, and Sushil K Singh* © 2020 Kumar S, et al. OPEN ACCESS Department of Cardiovascular and Thoracic Surgery, King George’s Medical University, India Keywords • Constrictive Pericarditis; Tuberculosis; Pericardiectomy; Surgical technique Abstract Introduction: Chronic constrictive pericarditis is a significant cause for diastolic dysfunction of the heart. Tuberculosis is considered a significant etiology in developing countries, whereas idiopathic cases are most common world-wide. Although pericardiectomy is an established procedure for chronic constrictive pericarditis, the extent of resection and utility of cardiopulmonary bypass is still debatable. The aim of this study was to study the feasibility and surgical outcomes of pericardiectomy for this disease in a large patient group in today’s scenario. Materials & methods: We retrospectively analyzed data of all patients who underwent pericardiectomy at our center between 2005 to 2019. We collected data of precise etiopathology of constrictive pericarditis. Analysis of surgical approach and outcomes was done. Inclusion criteria involved all consecutive patients with a diagnosis of constrictive pericarditis. Results: A total of 311 patients underwent pericardiectomy. Good surgical outcomes were demonstrated. There was a significant improvement in the functional status after surgery. Tuberculosis was the predominant etiology as seen in 48.23% cases, while idiopathic cases constituted 42.12%. -

Instructions and Data Element Definitions January 2004
Cardiac Surgery Report, Pediatrics (Under age 18) Form DOH-2254p Instructions and Data Element Definitions January 2004 NEW YORK STATE DEPARTMENT OF HEALTH BUREAU OF HOSPITAL & PRIMARY CARE SERVICES CARDIAC SERVICES PROGRAM One University Place, Suite 209 Rensselaer, NY 12144-3455 Phone: (518) 402-1016 Fax: (518) 402-6992 CARDIAC SERVICES PROGRAM CONTACTS Paula M. Waselauskas RN MSN, Administrator, [email protected] Casey S. Joseph MPH, Cardiac Initiatives Research Manager, [email protected] Table of Contents Topic Page Revision Highlights and Coding Clarification 3 When to Complete a Pediatric CSRS Form 4 ITEM-BY-ITEM INSTRUCTIONS PFI Number 5 Sequence Number 5 Patient Information Child’s Name 5 Medical Record Number 5 Child’s Social Security Number 5 Age in Years 5 Date of Birth 6 Sex 6 Ethnicity 6 Race 6 Residence Code 7 Hospital Admission Date 7 Primary Payer 7 Medicaid 7 Procedural Information Date of Surgery 7 Primary Surgeon Performing Surgery 8 Surgical Priority 8 Prior Surgery this Admission 8 Cardiac Diagnosis Code 8 Cardiac Procedure Code 9 Mode of Cardiopulmonary (CP) Bypass 9 Entire Procedure Off Pump 9 Minimally Invasive 9 CABG Information 10 Pre-Operative Status Pre-op Interventional Cath Procedure – this admission only 10 Weight at time of operation 11 Weight at Birth in grams 11 Pre-Operative conditions (None) 11 Previous Open Heart Operations 11 Previous Closed Heart Operations 12 Severe Cyanosis or Severe Hypoxia 12 Dialysis within 14 days prior to surgery 12 Any ventilator dependence during same admission