Aortic Valve Replacement Via a Right Parasternal Approach in a Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Surgical Management of Transcatheter Heart Valves
Corporate Medical Policy Surgical Management of Transcatheter Heart Valves File Name: surgica l_management_of_transcatheter_heart_valves Origination: 1/2011 Last CAP Review: 6/2021 Next CAP Review: 6/2022 Last Review: 6/2021 Description of Procedure or Service As the proportion of older adults increases in the U.S. population, the incidence of degenerative heart valve disease also increases. Aortic stenosis and mitra l regurgita tion are the most common valvular disorders in adults aged 70 years and older. For patients with severe valve disease, heart valve repair or replacement involving open heart surgery can improve functional status and qua lity of life. A variety of conventional mechanical and bioprosthetic heart valves are readily available. However, some individuals, due to advanced age or co-morbidities, are considered too high risk for open heart surgery. Alternatives to the open heart approach to heart valve replacement are currently being explored. Transcatheter heart valve replacement and repair are relatively new interventional procedures involving the insertion of an artificial heart valve or repair device using a catheter, rather than through open heart surgery, or surgical valve replacement (SAVR). The point of entry is typically either the femoral vein (antegrade) or femora l artery (retrograde), or directly through the myocardium via the apical region of the heart. For pulmonic and aortic valve replacement surgery, an expandable prosthetic heart valve is crimped onto a catheter and then delivered and deployed at the site of the diseased native valve. For valve repair, a small device is delivered by catheter to the mitral valve where the faulty leaflets are clipped together to reduce regurgitation. -

Severe Aortic Stenosis and the Valve Replacement Procedure
Severe Aortic Stenosis and the Valve Replacement Procedure A Guide for Patients and their Families If you’ve been diagnosed with severe aortic stenosis, you probably have a lot of questions and concerns. The information in this booklet will help you learn more about your heart, severe aortic stenosis, and treatment options. Your heart team will recommend which treatment option is best for you. Please talk with them about any questions you have. Table of Contents 4 About Your Heart 5 What Is Severe Aortic Stenosis? 5 What Causes Severe Aortic Stenosis? 7 What Are the Symptoms of Severe Aortic Stenosis? 8 Treatment Options for Severe Aortic Stenosis 10 Before a TAVR Procedure 12 What Are the Risks of TAVR? 2 3 About Your Heart What Is Severe See the difference between healthy and The heart is a muscle about the size of your fist. It is a pump that works nonstop to Aortic Stenosis? diseased valves send oxygen-rich blood throughout your entire body. The heart is made up of four The aortic valve is made up of two or three chambers and four valves. The contractions (heartbeats) of the four chambers push Healthy Valve the blood through the valves and out to your body. tissue flaps, called leaflets. Healthy valves open at every heart contraction, allowing blood to flow forward to the next chamber, and then close tightly to prevent blood from backing Pulmonic controls the flow of Aortic controls the flow of blood up. Blood flows in one direction only. This is Valve blood to the lungs Valve out of your heart to the important for a healthy heart. -
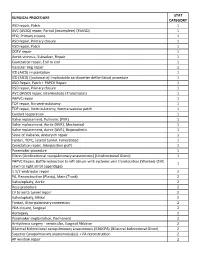
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon
r Me ula dic sc in a e V & f o S l u a Journal of Vascular r Nath et al., J Vasc Med Surg 2014, 2:2 g n r e u r y o DOI: 10.4172/2329-6925.1000135 J ISSN: 2329-6925 Medicine & Surgery Short Communication Open Access Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon Mridu Paban Nath1*, Malavika Barman2 and Rajib Kr Bhattacharrya3 1Assistant Professor, Department of Anesthesiology & Critical Care, Gauhati Medical College Hospital, Assam, India 2Assistant Professor, Department of Biochemistry, Tezpur Medical College Hospital, Assam, India 3Professor & Head, Department of Anesthesiology & Critical Care, FAA Medical College Hospital, Assam, India A 28 year old boy was referred from a private hospital for evaluation long periods of myocardial compression contributing to remodelling of constrictive pericarditis. He was diagnosed for the same about 4 of the ventricles and to greater involvement of the myocardium in years back with history of worsening shortness of breath and fatigue. patients who have undergone long periods of symptomatic pericardial At the time of presentation, patient required supplemental Oxygen constriction, as in our patient with a history of 4 years of symptoms. and was New York Heart Association Class-IV heart failure. Physical MacCaughan et al. [4] have described haemodynamic abnormalities examination revealed distension of jugular veins with significant after pericardiectomy in the largest series available (231 patients). The ascites & hepatomegaly. Bilateral pedal edema was absent; however investigators noted a 28% incidence of LCOS postoperatively in their patient was on long term therapy with loop diuretics. About 1 litre of patients, with many of the perioperative deaths occurring in this low abdominal paracentesis was done to relieve tense ascites. -

Surgery for Acquired Heart Disease
View metadata, citation and similar papers at core.ac.uk brought to you byCORE provided by Elsevier - Publisher Connector SURGERY FOR ACQUIRED HEART DISEASE EARLY RESULTS WITH PARTIAL LEFT VENTRICULECTOMY Patrick M. McCarthy, MD a Objective: We sought to determine the role of partial left ventriculectomy in Randall C. Starling, MD b patients with dilated cardiomyopathy. Methods: Since May 1996 we have James Wong, MBBS, PhD b performed partial left ventriculectomy in 53 patients, primarily (94%) in Gregory M. Scalia, MBBS b heart transplant candidates. The mean age of the patients was 53 years Tiffany Buda, RN a Rita L. Vargo, MSN, RN a (range 17 to 72 years); 60% were in class IV and 40% in class III. Marlene Goormastic, MPH c Preoperatively, 51 patients were thought to have idiopathic dilated cardio- James D. Thomas, MD b myopathy, one familial cardiomyopathy, and one valvular cardiomyopathy. Nicholas G. Smedira, MD a As our experience accrued we increased the extent of left ventriculectomy James B. Young, MD b and more complex mitral valve repairs. For two patients mitral valve replacement was performed. For 51 patients the anterior and posterior mitral valve leaflets were approximated (Alfieri repair); 47 patients also had ring posterior annuloplasty. In 27 patients (5!%) one or both papillary muscles were divided, additional left ventricular wall was resected, and the papillary muscle heads were reimplanted. Results: Echocardiography showed a significant decrease in left ventricular dimensions after resection (8.3 cm to 5.8 cm), reduction in mitral regurgitation (2.8+ to 0), and increase in forward ejection fraction (15.7% to 32.7%). -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G
ORIGINAL ARTICLES: CONGENITAL HEART SURGERY CONGENITAL HEART SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal. CONGENITAL HEART Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G. Co-Vu, MD,* Salil Ginde, MD,* Peter J. Bartz, MD, Peter C. Frommelt, MD, James S. Tweddell, MD, and Michael G. Earing, MD Department of Pediatrics, Division of Pediatric Cardiology, and Department of Internal Medicine, Division of Cardiovascular Medicine, and Department of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, Wisconsin Background. After the arterial switch operation (ASO) score increased at an average rate of 0.08 per year over for transposition of the great arteries (TGA), the native time after ASO. Freedom from neoaortic root dilation at pulmonary root and valve function in the systemic posi- 1, 5, 10, and 15 years after ASO was 84%, 67%, 47%, and tion, and the long-term risk for neoaortic root dilation 32%, respectively. Risk factors for root dilation include -pre ,(0.003 ؍ and valve regurgitation is currently undefined. The aim history of double-outlet right ventricle (p and length of ,(0.01 ؍ of this study was to determine the prevalence and pro- vious pulmonary artery banding (p Neoaortic valve regurgitation of at .(0.04 ؍ gression of neoaortic root dilation and neoaortic valve follow-up (p regurgitation in patients with TGA repaired with the least moderate degree was present in 14%. -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance....................................................................................................................... -

Evaluation of ECG Criteria for Left Ventricular Hypertrophy Before and After Aortic Valve Replacement Using Magnetic Resonance Imaging
MARCEL DEKKER, INC. • 270 MADISON AVENUE • NEW YORK, NY 10016 ©2003 Marcel Dekker, Inc. All rights reserved. This material may not be used or reproduced in any form without the express written permission of Marcel Dekker, Inc. JOURNAL OF CARDIOVASCULAR MAGNETIC RESONANCEw Vol. 5, No. 3, pp. 465–474, 2003 STRUCTURE AND FUNCTION Evaluation of ECG Criteria for Left Ventricular Hypertrophy Before and After Aortic Valve Replacement Using Magnetic Resonance Imaging Hugo P. Beyerbacht,1 Jeroen J. Bax,1,* Hildo J. Lamb,2 Arnoud van der Laarse,1 Hubert W. Vliegen,1 Albert de Roos,2 Aeilko H. Zwinderman,3 and Ernst E. van der Wall1 1Department of Cardiology, 2Department of Radiology, and 3Department of Medical Statistics, Leiden University Medical Centre, Leiden, The Netherlands ABSTRACT Purpose. Evaluation of different electrocardiographic criteria for left ventricular hypertrophy (ECG–LVH criteria) using left ventricular mass index (LVMI) determined by magnetic resonance imaging (MRI). In addition, the relation between LVMI regression after aortic valve replacement and corresponding ECG changes regarding LVH was studied. Methods. A group of 31 patients with severe aortic valve disease was studied to assess the presence of ECG–LVH and to measure LVMI and LV end-diastolic volume index (LVEDVI); 13 patients were restudied at 9.8 ^ 2.7 months after aortic valve replacement. Results. Three criteria had a sensitivity of 100% (SV1 þ RV5 or RV6 . 3.0 mV; SV1 or SV2 þ RV5 $ 3.5 mV; SV1 or SV2 þ RV5 or RV6 . 3.5 mV), at the cost of specificity (50%, 44.4% and 44.4%, respectively). -

Transcatheter Heart Valve Replacement
TRANSCATHETER ALI MASSUMI MD HEART VALVE NEIL E STRICKMAN MD REPLACEMENT 0 | P a g e Hall Garcia Cardiology Associates 6624 Fannin #2480 Houston, TX, USA 77030; +1-713-529-5530 ranscatheter Aortic Valve Replacement INTRODUCTION T The heart is a muscular organ located in your chest between your lungs which is designed to pump blood throughout the entire body. The right side of your heart pumps blood through the lungs, where the blood picks up oxygen. The left side of the heart receives this blood and pumps it to the rest of your body out through the AORTIC VALVE and into the circulation of the body. HEART CHAMBERS AND VALVES The heart is divided into four main areas, or chambers – two upper chambers (the left and right atrium) and two lower chambers (the left and right ventricle). These are the 4 valves which regulate the flow of blood through the heart, lungs and subsequently into the body’s circulation. They are called the aortic, mitral, pulmonary and tricuspid valves, whereas each is made of flaps of tissue called leaflets. See Figure 1 Figure 1 Hall Garcia Cardiology Associates 6624 Fannin #2480 Houston, TX, USA 77030; +1-713-529-5530 As the heart muscle contracts (squeezes), the valves open in one direction which allows the blood to circulate forward. When these valves close, the blood is prevented from flowing backward. There are 2 common problems that can develop in heart valves: VALVE STENOSIS This occurs when the valve is narrowed and does not completely open secondary to: o a build-up of calcium (mineral deposits) o high cholesterol (a waxy fat) o aging o genetics (such as a birth defect) VALVE INSUFFICIENCY / REGURGITATION This occurs when the valve does not fully close allowing blood to leak backward through the valve o Torn tendoniae (string-like architecture) o Ring dilation (degeneration like an automobile ring) AORTIC STENOSIS-(AS) Severe Aortic Valve Stenosis occurs when the narrowing of your aortic valve leaflets do not allow normal blood flow outward. -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty.