Log of ICD-9-CM and DRG Coding Updates and Revisions to PDI Documentation and Software
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Urological Trauma
Guidelines on Urological Trauma D. Lynch, L. Martinez-Piñeiro, E. Plas, E. Serafetinidis, L. Turkeri, R. Santucci, M. Hohenfellner © European Association of Urology 2007 TABLE OF CONTENTS PAGE 1. RENAL TRAUMA 5 1.1 Background 5 1.2 Mode of injury 5 1.2.1 Injury classification 5 1.3 Diagnosis: initial emergency assessment 6 1.3.1 History and physical examination 6 1.3.1.1 Guidelines on history and physical examination 7 1.3.2 Laboratory evaluation 7 1.3.2.1 Guidelines on laboratory evaluation 7 1.3.3 Imaging: criteria for radiographic assessment in adults 7 1.3.3.1 Ultrasonography 7 1.3.3.2 Standard intravenous pyelography (IVP) 8 1.3.3.3 One shot intraoperative intravenous pyelography (IVP) 8 1.3.3.4 Computed tomography (CT) 8 1.3.3.5 Magnetic resonance imaging (MRI) 9 1.3.3.6 Angiography 9 1.3.3.7 Radionuclide scans 9 1.3.3.8 Guidelines on radiographic assessment 9 1.4 Treatment 10 1.4.1 Indications for renal exploration 10 1.4.2 Operative findings and reconstruction 10 1.4.3 Non-operative management of renal injuries 11 1.4.4 Guidelines on management of renal trauma 11 1.4.5 Post-operative care and follow-up 11 1.4.5.1 Guidelines on post-operative management and follow-up 12 1.4.6 Complications 12 1.4.6.1 Guidelines on management of complications 12 1.4.7 Paediatric renal trauma 12 1.4.7.1 Guidelines on management of paediatric trauma 13 1.4.8 Renal injury in the polytrauma patient 13 1.4.8.1 Guidelines on management of polytrauma with associated renal injury 14 1.5 Suggestions for future research studies 14 1.6 Algorithms 14 1.7 References 17 2. -

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -
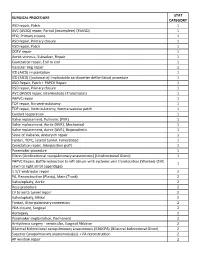
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Airway Obstruction After Biopsy by Cervical Mediastinoscopy in a Patient with a Mediastinal Mass -A Case Report
Korean J Anesthesiol 2012 July 63(1): 65-67 Case Report http://dx.doi.org/10.4097/kjae.2012.63.1.65 Airway obstruction after biopsy by cervical mediastinoscopy in a patient with a mediastinal mass -A case report- Yong-Cheol Lee2, Sang-Jin Park1, and In-seong Kim1 Department of Anesthesiology and Pain Medicine, 1College of Medicine, Yeungnam University, 2School of Medicine, Keimyung University, Daegu, Korea Biopsy, using mediastinoscopy is commonly employed for accurate histologic diagnosis of a mediastinal mass. However, since the mass is not removed during the procedure, it may cause compression of vital structures such as major airways, the heart, the pulmonary artery, and the superior vena cava after surgery. We observed a case of a 66-year-old man with a mediastinal mass that caused severe airway obstruction during recovery from anesthesia following mediastinoscopic biopsy, probably caused by upper airway edema which seemed to originate from compression of the superior vena cava. Therefore, we suggest that unexpected airway obstruction in a patient with a mediastinal mass can be due to superior vena cava compression. (Korean J Anesthesiol 2012; 63: 65-67) Key Words: Airway obstruction, Mediastinoscopy, Superior vena cava. Biopsies, using mediastinoscopy are commonly utilized Case Report to decide on the treatment of mediastinal masses. However, since the mass is not removed during the biopsy, it may cause A 66-year-old man visited the hospital with a one month compression of vital structures after the procedure [1]. The history of chest discomfort and sporadic swelling in the face superior vena cava has an especially low intravascular pressure and arms in the morning. -

Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon
r Me ula dic sc in a e V & f o S l u a Journal of Vascular r Nath et al., J Vasc Med Surg 2014, 2:2 g n r e u r y o DOI: 10.4172/2329-6925.1000135 J ISSN: 2329-6925 Medicine & Surgery Short Communication Open Access Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon Mridu Paban Nath1*, Malavika Barman2 and Rajib Kr Bhattacharrya3 1Assistant Professor, Department of Anesthesiology & Critical Care, Gauhati Medical College Hospital, Assam, India 2Assistant Professor, Department of Biochemistry, Tezpur Medical College Hospital, Assam, India 3Professor & Head, Department of Anesthesiology & Critical Care, FAA Medical College Hospital, Assam, India A 28 year old boy was referred from a private hospital for evaluation long periods of myocardial compression contributing to remodelling of constrictive pericarditis. He was diagnosed for the same about 4 of the ventricles and to greater involvement of the myocardium in years back with history of worsening shortness of breath and fatigue. patients who have undergone long periods of symptomatic pericardial At the time of presentation, patient required supplemental Oxygen constriction, as in our patient with a history of 4 years of symptoms. and was New York Heart Association Class-IV heart failure. Physical MacCaughan et al. [4] have described haemodynamic abnormalities examination revealed distension of jugular veins with significant after pericardiectomy in the largest series available (231 patients). The ascites & hepatomegaly. Bilateral pedal edema was absent; however investigators noted a 28% incidence of LCOS postoperatively in their patient was on long term therapy with loop diuretics. About 1 litre of patients, with many of the perioperative deaths occurring in this low abdominal paracentesis was done to relieve tense ascites. -

Update in Anaesthesia
Update in Anaesthesia Pulmonary Function Tests and Assessment for Lung Resection David Portch*, Bruce McCormick *Correspondence Email: [email protected] INTRODUCTION Summary respectively. There are 2400 lobectomies and 500 The aim of this article is to describe the tests available This article describes the for the assessment of patients presenting for lung pneumonectomies performed in the UK each year, steps taken to evaluate resection. The individual tests are explained and we with in-hospital mortality 2-4% for lobectomy and patients’ fitness for lung 4 describe how patients may progress through a series of 6-8% for pneumonectomy. resection surgery. Examples tests to identify those amenable to lung resection. Lung resection is most frequently performed to treat are used to demonstrate interpretation of these tests. Pulmonary function testing is a vital part of the non-small cell lung cancer. This major surgery places It is vital to use these tests in assessment process for thoracic surgery. However, large metabolic demands on patients, increasing conjunction with a thorough for other types of surgery there is no evidence postoperative oxygen consumption by up to 50%. history and examination that spirometry is more effective than history and Patients presenting for lung resection are often high in order to achieve an examination in predicting postoperative pulmonary risk due to a combination of their age (median age accurate assessment of each complications in patients with known chronic lung is 70 years)5 and co-morbidities. Since non-surgical patient’s level of function. conditions. Furthermore specific spirometric values mortality approaches 100%, a thorough assessment of Much of this assessment (e.g. -

Delineation of Privileges Urology Privileges Provider Name
Delineation Of Privileges Urology Privileges Provider Name: Privilege Requested Deferred Approved UROLOGY PRIVILEGES Criteria - New Applicants:: Board Certification or qualified for certification by the American Board of Urology. Criteria - Current Staff Members Only: Successful completion of an ACGME or AOA approved training program; OR demonstrated acceptable practice in the privileges being requested for a minimum of five (5) years. Proctoring Requirements: A minimum of eight (8) cases, in accordance with the Medical Staff Proctoring Protocol. GENERAL PRIVILEGES: Admit ___ ___ ___ Consultation Only Privileges ___ ___ ___ Surgical Assist Only ___ ___ ___ Local block anesthesia ___ ___ ___ Regional block anesthesia ___ ___ ___ Sedation analgesia ___ ___ ___ Criteria: Requires successful completion of the Sedation Assessment test. Additional criteria effective April 1, 2015: a) Evidence of current ACLS and/or PALS certification from the American Heart Association; AND b) Evidence of completion of an Airway Management Course a) Adult Sedation ___ ___ ___ b) Pediatric Sedation (17 years and under) ___ ___ ___ CATEGORY 1 - UROLOGY PRIVILEGES ___ ___ ___ Includes the management and coordination of care, treatment and services, including: medical history and physical evaluations, consultations and prescribing medication in accordance with DEA certificate. Urethral, bladder catheterization ___ ___ ___ Suprapubic, bladder aspiration ___ ___ ___ Page 1 Printed on Wednesday, December 10, 2014 Delineation Of Privileges Urology Privileges Provider -

A Systematic Review of Graft Augmentation Urethroplasty Techniques for the Treatment of Anterior Urethral Strictures
EUROPEAN UROLOGY 59 (2011) 797–814 available at www.sciencedirect.com journal homepage: www.europeanurology.com Review – Reconstructive Urology A Systematic Review of Graft Augmentation Urethroplasty Techniques for the Treatment of Anterior Urethral Strictures Altaf Mangera *, Jacob M. Patterson, Christopher R. Chapple Royal Hallamshire Hospital, Sheffield, United Kingdom Article info Abstract Article history: Context: Reconstructive surgeons who perform urethroplasty have a variety of Accepted February 2, 2011 techniques in their armamentarium that may be used according to factors such as Published online ahead of aetiology, stricture position, and length. No one technique is recommended. print on February 11, 2011 Objective: Our aim was to assess the reported outcomes of the various techniques for graft augmentation urethroplasty according to site of surgery. Keywords: Evidence acquisition: We performed an updated systematic review of the Medline Augmentation urethroplasty literature from 1985 to date and classified the data according to the site of surgery Anterior urethral stricture and technique used. Data are also presented on the type of graft used and the Bulbar urethroplasty follow-up methodology used by each centre. Dorsal onlay bulbar Evidence synthesis: More than 2000 anterior urethroplasty procedures have been urethroplasty describedinthe literature.Whenconsidering the bulbar urethra there isnosignificant Ventral onlay bulbar difference between the average success rates of the dorsal and the ventral onlay urethroplasty procedures, 88.4% and 88.8% at42.2 and 34.4 moin 934 and 563 patients,respectively. Penile urethroplasty The lateral onlay technique has only been described in six patients and has a reported success rate of 83% at 77 mo. The Asopa and Palminteri techniques have been described in 89 and 53 patients with a success rate of 86.7% and 90.1% at 28.9 and 21.9 mo, respectively. -

Consider a Minor Surgery As a Major Surgery and Order Preoperative Tests Accordingly (I.E
ROUTINE PREOPERATIVE LAB TEST GUIDELINES For adult patients (≥ 16 years) undergoing elective surgery TESTS WITHIN 6 MONTHS OF SURGERY CLINICAL JUDGEMENT IS REQUIRED EXCLUSIONS are valid, provided there has been no interim change as additional tests may be appropriate for patients with this guideline does not apply to patients in the patient’s condition. complex or uncommon surgical or medical conditions. undergoing cardiac surgery or cesarean section. MINOR SURGERY MAJOR SURGERY Associated with an expected blood loss of Associated with an expected blood loss of >500mL, significant fluid shifts and typically, at least one night in <500mL, minimal fluid shifts and is typically hospital*. Includes laparoscopic surgery (except cholecystectomy and tubal ligation); open resection of organs; done on an ambulatory basis (day surgery/ large joint replacements; mastectomy with reconstruction; and spine, thoracic, vascular, or intracranial surgery. same day discharge)*. It includes cataract * If the surgery is typically ambulatory but the patient has a medical or social reason for overnight admission (i.e. OSA, no support surgery; breast surgery without reconstruction; at home), still consider the surgery minor in determining which lab tests to order. laparoscopic cholecystectomy and tubal ligation; and most cutaneous, superficial, All Major Age: 16-49 years old Age: 50+ years old endoscopic and arthroscopic procedures. or Order • add ECG for patients with DM, HTN, Renal, • add ECG + + – DO NOT ORDER PREOP TESTS CBC Cardiovascular or severe Respiratory disease. + + – • add Na , K , Cl , TCO2 including: chest x-rays, Na , K , Cl , TCO , + + – 2 Na K Cl TCO & Cr/ eGFR serum glucose, CBC, ECG, INR, urinalysis, • add , , , 2 for patients with • add Cr/ eGFR renal, liver or thyroid function tests in DM; HTN; Malnutrition; BMI > 40; Renal, Liver or asymptomatic** patients. -

(Part 1): Management of Male Urethral Stricture Disease
EURURO-9412; No. of Pages 11 E U R O P E A N U R O L O G Y X X X ( 2 0 2 1 ) X X X – X X X ava ilable at www.sciencedirect.com journa l homepage: www.europeanurology.com Review – Reconstructive Urology European Association of Urology Guidelines on Urethral Stricture Disease (Part 1): Management of Male Urethral Stricture Disease a, b c d Nicolaas Lumen *, Felix Campos-Juanatey , Tamsin Greenwell , Francisco E. Martins , e f a c g Nadir I. Osman , Silke Riechardt , Marjan Waterloos , Rachel Barratt , Garson Chan , h i a j Francesco Esperto , Achilles Ploumidis , Wesley Verla , Konstantinos Dimitropoulos a b Division of Urology, Gent University Hospital, Gent, Belgium; Urology Department, Marques de Valdecilla University Hospital, Santander, Spain; c d Department of Urology, University College London Hospital, London, UK; Department of Urology, Santa Maria University Hospital, University of Lisbon, e f Lisbon, Portugal; Department of Urology, Sheffield Teaching Hospitals, Sheffield, UK; Department of Urology, University Medical Center Hamburg- g h Eppendorf, Hamburg, Germany; Division of Urology, University of Saskatchewan, Saskatoon, Canada; Department of Urology, Campus Biomedico i j University of Rome, Rome, Italy; Department of Urology, Athens Medical Centre, Athens, Greece; Aberdeen Royal Infirmary, Aberdeen, UK Article info Abstract Article history: Objective: To present a summary of the 2021 version of the European Association of Urology (EAU) guidelines on management of male urethral stricture disease. Accepted May 15, 2021 Evidence acquisition: The panel performed a literature review on these topics covering a time frame between 2008 and 2018, and used predefined inclusion and exclusion criteria Associate Editor: for the literature to be selected. -

Mediastinoscopy: a Clinical Evaluation of 400 Consecutive Cases
Thorax: first published as 10.1136/thx.24.5.585 on 1 September 1969. Downloaded from Thorax (1969), 24, 585. Mediastinoscopy: A clinical evaluation of 400 consecutive cases C. L. SARIN1 AND H. C. NOHL-OSER From the Thoracic Surgical Unit, Harefield Hospital, Harefield, Middlesex Mediastinoscopy was carried out in 400 cases, including 296 of bronchogenic carcinoma. At the time of presentation the new growth had already spread to involve the mediastinal lymph nodes in slightly more than 50% of these. The incidence of involvement was 76% in oat-cell and 35% in squamous-cell carcinoma. Non-resectability at thoracotomy was encountered in seven out of 120 patients. We advocate this procedure in every case of bronchogenic carcinoma which is considered operable on other counts. In patients in whom the mediastinal lymph nodes are invaded by growth we prefer radical radiotherapy to surgery, as the long-term survival of the two methods is comparable. This procedure may be the only source of positive histological proof of diagnosis, not only in carcinoma but in other types of intrathoracic disease. We believe that this procedure reduces the number of unnecessary exploratory thoracotomies. Carlens (1959) introduced diagnostic exploration sible. Biopsy in such cases can be obtained from tissues inside the thoracic inlet. in the of the superior mediastinum. The space explored just Bleeding, copyright. is part of the superior mediastinum which is presence of incipient or developed superior vena caval of the obstruction, or dense fibrosis of the pre-tracheal situated around tihe intrathoracic part fascia, can make the procedure difficult or impossible.