Hodgkin's Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications on the Basic Skeletons of Vinblastine and Vincristine
Molecules 2012, 17, 5893-5914; doi:10.3390/molecules17055893 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Modifications on the Basic Skeletons of Vinblastine and Vincristine Péter Keglevich, László Hazai, György Kalaus and Csaba Szántay * Department of Organic Chemistry and Technology, University of Technology and Economics, H-1111 Budapest, Szt. Gellért tér 4, Hungary * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +36-1-463-1195; Fax: +36-1-463-3297. Received: 30 March 2012; in revised form: 9 May 2012 / Accepted: 10 May 2012 / Published: 18 May 2012 Abstract: The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties. Keywords: antitumor therapy; vinblastine; vincristine; derivatives 1. Introduction Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents. In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase. Molecules 2012, 17 5894 Figure 1. -
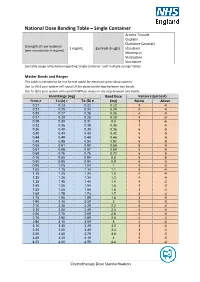
National Dose Banding Table – Single Container
National Dose Banding Table – Single Container Arsenic Trioxide Cisplatin Cladribine (Leustat) Strength of raw material 1 mg/mL Example drug(s) Idarubicin (after reconstitution if required) Mitomycin Vinblastine Vincristine See table usage notes below regarding ‘single container’ and ‘multiple syringe’ tables. Master Bands and Ranges This table is intended to be in a format useful for electronic prescribing systems. Use To (A) if your system will round UP for doses on the step between two bands. Use To (B) if your system will round DOWN for doses on the step between two bands. Band Range (mg) Band Dose Variance (percent) From ≥ To (A) < To (B) ≤ (mg) Below Above 0.21 0.23 0.22 0.22 5 -4 0.23 0.25 0.24 0.24 4 -4 0.25 0.27 0.26 0.26 4 -4 0.27 0.29 0.28 0.28 4 -3 0.29 0.32 0.31 0.3 3 -6 0.32 0.36 0.35 0.34 7 -5 0.36 0.40 0.39 0.38 6 -5 0.40 0.44 0.43 0.42 5 -5 0.44 0.49 0.48 0.46 5 -6 0.49 0.55 0.54 0.52 6 -5 0.55 0.61 0.60 0.58 6 -5 0.61 0.68 0.67 0.64 5 -6 0.68 0.76 0.75 0.72 6 -5 0.76 0.85 0.84 0.8 5 -6 0.85 0.95 0.94 0.9 6 -5 0.95 1.05 1.04 1 5 -5 1.05 1.15 1.14 1.1 5 -4 1.15 1.25 1.24 1.2 4 -4 1.25 1.35 1.34 1.3 4 -4 1.35 1.45 1.44 1.4 4 -3 1.45 1.55 1.54 1.5 4 -3 1.55 1.65 1.64 1.6 3 -3 1.65 1.75 1.74 1.7 3 -3 1.75 1.90 1.89 1.8 3 -5 1.90 2.10 2.09 2 5 -5 2.10 2.30 2.29 2.2 5 -4 2.30 2.50 2.49 2.4 4 -4 2.50 2.70 2.69 2.6 4 -4 2.70 2.90 2.89 2.8 4 -3 2.90 3.10 3.09 3 4 -3 3.10 3.30 3.29 3.2 3 -3 3.30 3.50 3.49 3.4 3 -3 3.50 3.80 3.79 3.6 3 -5 3.80 4.20 4.19 4 5 -5 4.20 4.60 4.59 4.4 5 -4 Chemotherapy Dose Standardisation Band Range (mg) -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 2)
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 2) The selection, dosing, and administration of anticancer agents and the management of associated toxicities are complex. Drug dose modifications and schedule and initiation of supportive care interventions are often necessary because of expected toxicities and because of individual patient variability, prior treatment, and comorbidities. Thus, the optimal delivery of anticancer agents requires a healthcare delivery team experienced in the use of such agents and the management of associated toxicities in patients with cancer. The cancer treatment regimens below may include both FDA-approved and unapproved uses/regimens and are provided as references only to the latest treatment strategies. Clinicians must choose and verify treatment options based on the individual patient. NOTE: GREY SHADED BOXES CONTAIN UPDATED REGIMENS. REGIMEN DOSING Classical Hodgkin Lymphoma—First-Line Treatment General treatment note: Routine use of growth factors is not recommended. Leukopenia is not a factor for treatment delay or dose reduction (except for escalated BEACOPP).1 CR=complete response IPS=International Prognostic Score PD=progressive disease PFTs=pulmonary function tests PR=partial response RT=radiation therapy SD=stable disease Stage IA, IIA Favorable ABVD (doxorubicin [Adriamycin] Days 1 and 15: Doxorubicin 25mg/m2 IV + bleomycin 10mg/m2 IV + vinblastine + bleomycin + vinblastine + 6mg/m2 IV + dacarbazine 375mg/m2 IV. dacarbazine [DTIC-Dome]) + Repeat cycle every 4 weeks for 2–4 cycles. involved-field radiotherapy (IFRT)1–4 Follow with IFRT after completion of chemotherapy. Abbreviated Stanford V Weeks 1, 3, 5 and 7: Vinblastine 6mg/m2 IV + doxorubicin 25mg/m2 IV. (doxorubicin + vinblastine + Weeks 1 and 5: Mechlorethamine 6mg/m2. -

Hodgkin Lymphoma
Hodgkin Lymphoma Erica, Hodgkin lymphoma survivor Revised 2016 Publication Update Hodgkin Lymphoma The Leukemia & Lymphoma Society wants you to have the most up-to-date information about blood cancer treatment. See below for important new information that was not available at the time this publication was printed. In May 2017, the Food and Drug Administration (FDA) approved nivolumab (Opdivo®) for the treatment of adult patients with classical Hodgkin lymphoma (HL) that has relapsed or progressed after 3 or more lines of systemic therapy that includes autologous hematopoietic stem cell transplantation (HSCT). It is also approved for the treatment of adult patients with classical HL that has relapsed or progressed after autologous HSCT and brentuximab vedotin. These indications are approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. In March 2017, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda®) for the treatment of adult and pediatric patients with refractory classical Hodgkin lymphoma (cHL), or who have relapsed after 3 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials. For more information, contact an Information Specialist at (800) 955-4572 or [email protected]. Information Specialists: 800.955.4572 I www.LLS.org PS57 A Message from Louis J. DeGennaro, PhD President and CEO of The Leukemia & Lymphoma Society The Leukemia & Lymphoma Society (LLS) is the world’s largest voluntary health organization dedicated to finding cures for blood cancer patients. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Desmoid Tumors: Understanding Treatment Options in 2019
Desmoid Tumors: Understanding Treatment Options in 2019 Breelyn A. Wilky, MD Associate Professor Sarcoma Program, Division of Oncology A real desmoid story… 35 year old female physician who noted abdominal cramping and pain in her mid abdomen and a palpable mass in August 2018 She performed an ultrasound on herself in the ER which showed a 4.5 x 4.5 cm mass in her mid abdomen CT scan showed a mesenteric mass that was wrapped around and blocking the inferior mesenteric vein with multiple satellite nodules/lymph nodes. Biopsy confirmed the diagnosis of desmoid tumor A real desmoid story… Mother had a neuroendocrine cancer in the appendix, father had Hodgkins lymphoma The patient had a negative colonoscopy for any polyps She had no other medical problems but has three children, with the last baby born 4 months prior to her symptoms. This most recent baby was conceived with in vitro fertilization. Tumor board discussion… Ongoing pain likely related to venous outflow obstruction (confirmed on colonoscopy as well) Treatment options in 2019… Watch and Wait • Doxorubicin/ Dacarbazine Chemotherapy Attempt • MTX/vinblastine (IV) Surgery • Doxil • Sorafenib • Imatinib Targeted • Pazopanib treatments Radiation • Nirogacestat (chemo pills) • Tegavivint (IV) • Tamoxifen +/- sulindac Estrogen- Ablation • Aromatase blocking IRE/Nanoknife inhibitors Treatments HIFU • Celebrex Choosing a treatment approach . Anxiety over “doing nothing” . How long is acceptable to wait for Symptoms a response? Location . Can shrinkage or necrosis create a Risk to surrounding structures more definitive option? (surgery, and organs electroporation?) Growth pattern over time . What is the expected function or appearance after my desmoid Obvious estrogen exposure surgery? (pregnancy) . -

Older Patients
Older patients (aged ≥60 years) with previously untreated advanced-stage classical Hodgkin lymphoma: a detailed analysis from the phase III ECHELON-1 study by Andrew M. Evens, Joseph M. Connors, Anas Younes, Stephen M. Ansell, Won Seog Kim, John Radford, Tatyana Feldman, Joseph Tuscano, Kerry J. Savage, Yasuhiro Oki, Andrew Grigg, Christopher Pocock, Monika Dlugosz-Danecka, Keenan Fenton, Andres Forero-Torres, Rachael Liu, Hina Jolin, Ashish Gautam, and Andrea Gallamini Haematologica 2021 [Epub ahead of print] Citation: Andrew M. Evens, Joseph M. Connors, Anas Younes, Stephen M. Ansell, Won Seog Kim, John Radford, Tatyana Feldman, Joseph Tuscano, Kerry J. Savage, Yasuhiro Oki, Andrew Grigg, Christopher Pocock, Monika Dlugosz-Danecka, Keenan Fenton, Andres Forero-Torres, Rachael Liu, Hina Jolin, Ashish Gautam, and Andrea Gallamini. Older patients (aged ≥60 years) with previously untreated advanced-stage classical Hodgkin lymphoma: a detailed analysis from the phase III ECHELON-1 study. Haematologica. 2021; 106:xxx doi:10.3324/haematol.2021.278438 Publisher's Disclaimer. E-publishing ahead of print is increasingly important for the rapid dissemination of science. Haematologica is, therefore, E-publishing PDF files of an early version of manuscripts that have completed a regular peer review and have been accepted for publication. E-publishing of this PDF file has been approved by the authors. After having E-published Ahead of Print, manuscripts will then undergo technical and English editing, typesetting, proof correction and be presented for the authors' final approval; the final version of the manuscript will then appear in print on a regular issue of the journal. All legal disclaimers that apply to the journal also pertain to this production process. -

Procarbazine
Procarbazine DRUG NAME: Procarbazine SYNONYM(S): COMMON TRADE NAME(S): MATULANE® CLASSIFICATION: alkylating agent Special pediatric considerations are noted when applicable, otherwise adult provisions apply. MECHANISM OF ACTION: Procarbazine is a cell cycle phase-nonspecific1 pro-drug and derivative of hydrazine whose mechanism of action has not yet been clearly defined. Procarbazine may act by inhibiting protein, RNA, and DNA synthesis,2-4 and by causing free-radical damage to DNA and inhibition of mitosis.3,5 Procarbazine also has monoamine oxidase (MAO) inhibiting properties2,3 and is an immunosuppressive agent.2 Cross resistance with other chemotherapy agents has not been demonstrated.2 PHARMACOKINETICS: Oral Absorption rapid and complete; peak plasma concentration in 1 h Distribution rapid distribution including into liver, kidneys, intestinal wall, and skin3 cross blood brain barrier? yes volume of distribution no information found plasma protein binding no information found Metabolism complex spontaneous chemical decomposition and biotransformation to active metabolites,3,6 primarily in the liver3 via cytochrome P450 oxidoreductase and mitochondrial monoamine oxidase7 active metabolite(s)3,5,6,8 yes; including azo-and methylazoxy-metabolites and hydrogen peroxide inactive metabolite(s)2,9 yes; including N-isopropyl-terephthalmamic acid Excretion primarily hepatic with some renal2,3 and pulmonary10 elimination urine2-4 25-70% in 24 h primarily as N-isopropyl- terephthalmamic acid; <5-20% unchanged feces7 minimal terminal half life3,4 -

Methotrexate / Vincristine / Leucovorin / Procarbazine (Cycles 1,3,5)
Protocol Index IP DEANGELIS WITH RITUXIMAB - METHOTREXATE / VINCRISTINE / LEUCOVORIN / PROCARBAZINE (CYCLES 1,3,5) Types: ONCOLOGY TREATMENT Synonyms: PRIMARY, CENTRAL, CNS, LYMPH, MTX, ONCOV, METHOT, VINCR, PROCARB, DEANGELIS, DEAN, RITUX Cycle 1 Repeat 1 time Cycle length: 14 days Day 1 Perform every 1 day x1 Labs ☑ COMPREHENSIVE METABOLIC PANEL Interval: Once Occurrences: -- ☑ CBC WITH PLATELET AND DIFFERENTIAL Interval: Once Occurrences: -- ☑ MAGNESIUM LEVEL Interval: Once Occurrences: -- ☑ LDH Interval: Once Occurrences: -- ☑ URIC ACID LEVEL Interval: Once Occurrences: -- ☑ PHOSPHORUS LEVEL Interval: Once Occurrences: -- Labs ☑ METHOTREXATE LEVEL Interval: Once Occurrences: -- ☑ PH, URINALYSIS Interval: Conditional Occurrences: -- Frequency Comments: Draw prior to starting Methotrexate and PRN until pH GREATER than 7. Then draw urine pH every day until MTX is LESS than 0.05 Provider Communication ONC PROVIDER COMMUNICATION 58 Interval: Once Occurrences: -- Comments: Prior to beginning Rituxan infusion, please check if a Hepatitis B and C serology has been performed within the past 6 months. Hepatitis B and C serologies results: Push F2:11554001 drawn on ***. Provider Communication ONC PROVIDER COMMUNICATION 5 Interval: Once Occurrences: -- Comments: Use baseline weight to calculate dose. Adjust dose for weight gains/losses of greater than or equal to 10%. Provider Communication ONC PROVIDER COMMUNICATION 12 Interval: Until Occurrences: -- discontinued Comments: Careful monitoring of pulmonary function tests should be performed prior -

VINBLASTINE-VINCRISTINE (Chlvpp-EVA
Chemotherapy Protocol LYMPHOMA CHLORAMBUCIL-DOXORUBICIN-ETOPOSIDE-PREDNISOLONE-PROCARBAZINE- VINBLASTINE-VINCRISTINE (ChlVPP-EVA) Regimen • Lymphoma – ChlVPP-EVA-Chlorambucil-Doxorubicin-Etoposide-Prednisolone- Procarbazine-Vinblastine-Vincristine Indication • Hodgkin’s Lymphoma Toxicity Drug Adverse Effect Chlorambucil Gastro-intestinal disturbance Doxorubicin Cardiomyopathy, alopecia, urinary discolouration (red) Etoposide Hypotension on rapid infusion, hyperbilirubinaemia Weight gain, gastro-intestinal disturbances, hyperglycaemia, Prednisolone CNS disturbances, cushingoid changes, glucose intolerance Procarbazine Insomnia, ataxia, hallucinations, headache Vinblastine Peripheral neuropathy, constipation, jaw pain, ileus Vincristine Peripheral neuropathy, constipation, jaw pain, ileus The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Patients diagnosed with Hodgkin’s Lymphoma carry a lifelong risk of transfusion-associated graft versus host disease (TA-GVHD). Where blood products are required these patients must receive only irradiated blood products for life. Local blood transfusion departments must be notified as soon as a diagnosis is made and the patient must be issued with an alert card to carry with them at all times. Monitoring Drugs • FBC, LFTs and U&Es prior to day one and eight of treatment Version 1 (May 2018) Page 1 of 10 Lymphoma- ChlVPP-EVA-Chlorambucil-Doxorubicin-Etoposide-Prednisolone-Procarbazine-Vinblastine-Vincristine Dose Modifications The dose modifications listed are for haematological, liver and renal function and limited drug specific toxicities. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re-escalated in subsequent cycles without consultant approval. It is also a general rule for chemotherapy that if a third dose reduction is necessary treatment should be stopped.