“Mining Magnetite for Your Future.”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Study of Earth's Magnetism
THE STUDY OF EARTH’S MAGNETISM (1269-1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM Vincent Courtillot, Jean-Louis Le Mouël To cite this version: Vincent Courtillot, Jean-Louis Le Mouël. THE STUDY OF EARTH’S MAGNETISM (1269-1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM. Reviews of Geophysics, American Geophysical Union, 2007, 45 (3), pp.RG3008. 10.1029/2006RG000198. insu-02448801 HAL Id: insu-02448801 https://hal-insu.archives-ouvertes.fr/insu-02448801 Submitted on 22 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THE STUDY OF EARTH’S MAGNETISM (1269–1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM Vincent Courtillot1 and Jean-Louis Le Moue¨l1 Received 17 February 2006; revised 14 July 2006; accepted 18 September 2006; published 25 September 2007. [1] This paper summarizes the histories of geomagnetism induced magnetizations), Delesse (remagnetization in a and paleomagnetism (1269–1950). The role of Peregrinus direction opposite to the original), and Melloni (direction of is emphasized. In the sixteenth century a debate on local lava magnetization acquired at time of cooling). -

Coulsonite Fev2o4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia
minerals Article Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia Alena A. Kompanchenko Geological Institute of the Federal Research Centre “Kola Science Centre of the Russian Academy of Sciences”, 14 Fersman Street, 184209 Apatity, Russia; [email protected]; Tel.: +7-921-048-8782 Received: 24 August 2020; Accepted: 21 September 2020; Published: 24 September 2020 Abstract: This work presents new data on a rare vanadium spinel group mineral, i.e., coulsonite FeV2O4 established in massive sulfide ores of the Bragino occurrence in the Kola region, Russia. Coulsonite in massive sulfide ores of the Bragino occurrence is one of the most common vanadium minerals. Three varieties of coulsonite were established based on its chemical composition, some physical properties, and mineral association: coulsonite-I, coulsonite-II, and coulsonite-III. Coulsonite-I forms octahedral crystal clusters of up to 500 µm, and has a uniformly high content of 2 Cr2O3 (20–30 wt.%), ZnO (up to 4.5 wt.%), and MnO (2.8 wt.%), high microhardness (743 kg/mm ) and coefficient of reflection. Coulsonite-II was found in relics of quartz–albite veins in association with other vanadium minerals. Its features are a thin tabular shape and enrichment in TiO2 of up to 18 wt.%. Coulsonite-III is the most common variety in massive sulfide ores of the Bragino occurrence. Coulsonite-III forms octahedral crystals of up to 150 µm, crystal clusters, and intergrowths with V-bearing ilmenite, W-V-bearing rutile, Sc-V-bearing senaite, etc. Chemical composition of coulsonite-III is characterized by wide variation of the major compounds—Fe, V, Cr. -

Chromite Crystal Structure and Chemistry Applied As an Exploration Tool
Western University Scholarship@Western Electronic Thesis and Dissertation Repository February 2015 Chromite Crystal Structure and Chemistry applied as an Exploration Tool Patrick H.M. Shepherd The University of Western Ontario Supervisor Dr. Roberta L. Flemming The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Patrick H.M. Shepherd 2015 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Geology Commons Recommended Citation Shepherd, Patrick H.M., "Chromite Crystal Structure and Chemistry applied as an Exploration Tool" (2015). Electronic Thesis and Dissertation Repository. 2685. https://ir.lib.uwo.ca/etd/2685 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Western University Scholarship@Western University of Western Ontario - Electronic Thesis and Dissertation Repository Chromite Crystal Structure and Chemistry Applied as an Exploration Tool Patrick H.M. Shepherd Supervisor Roberta Flemming The University of Western Ontario Follow this and additional works at: http://ir.lib.uwo.ca/etd Part of the Geology Commons This Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in University of Western Ontario - Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Chromite Crystal Structure and Chemistry Applied as an Exploration Tool (Thesis format: Integrated Article) by Patrick H.M. -

The Efficient Improvement of Original Magnetite in Iron Ore Reduction
minerals Article The Efficient Improvement of Original Magnetite in Iron Ore Reduction Reaction in Magnetization Roasting Process and Mechanism Analysis by In Situ and Continuous Image Capture Bing Zhao 1,2, Peng Gao 1,2,*, Zhidong Tang 1,2 and Wuzhi Zhang 1,2 1 School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China; [email protected] (B.Z.); [email protected] (Z.T.); [email protected] (W.Z.) 2 National-Local Joint Engineering Research Center of High-Efficient Exploitation Technology for Refractory Iron Ore Resources, Shenyang 110819, China * Correspondence: [email protected]; Tel.: +86-024-8368-8920 Abstract: Magnetization roasting followed by magnetic separation is considered an effective method for recovering iron minerals. As hematite and magnetite are the main concomitant constituents in iron ores, the separation index after the magnetization roasting will be more optimized than with only hematite. In this research, the mechanism of the original magnetite improving iron ore reduction during the magnetization roasting process was explored using ore fines and lump ore samples. Under optimum roasting conditions, the iron grade increased from 62.17% to 65.22%, and iron recovery increased from 84.02% to 92.02% after separation, when Fe in the original magnetite content increased from 0.31% to 8.09%, although the Fe masses in each sample were equal. For lump ores with magnetite and hematite intergrowth, the method of in situ and continuous image capture Citation: Zhao, B.; Gao, P.; Tang, Z.; for microcrack generation and the evolution of the magnetization roasting process was innovatively Zhang, W. -

Recovery of Magnetite-Hematite Concentrate from Iron Ore Tailings
E3S Web of Conferences 247, 01042 (2021) https://doi.org/10.1051/e3sconf/202124701042 ICEPP-2021 Recovery of magnetite-hematite concentrate from iron ore tailings Mikhail Khokhulya1,*, Alexander Fomin1, and Svetlana Alekseeva1 1Mining Institute of Kola Science Center of Russian Academy of Sciences, Apatity, 184209, Russia Abstract. The research is aimed at study of the probable recovery of iron from the tailings of the Olcon mining company located in the north-western Arctic zone of Russia. Material composition of a sample from a tailings dump was analysed. The authors have developed a separation production technology to recover magnetite-hematite concentrate from the tailings. A processing flowsheet includes magnetic separation, milling and gravity concentration methods. The separation technology provides for production of iron ore concentrate with total iron content of 65.9% and recovers 91.0% of magnetite and 80.5% of hematite from the tailings containing 20.4% of total iron. The proposed technology will increase production of the concentrate at a dressing plant and reduce environmental impact. 1 Introduction The mineral processing plant of the Olcon JSC, located at the Murmansk region, produces magnetite- At present, there is an important problem worldwide in hematite concentrate. The processing technology the disposal of waste generated during the mineral includes several magnetic separation stages to produce production and processing. Tailings dumps occupy huge magnetite concentrate and two jigging stages to produce areas and pollute the environment. However, waste hematite concentrate from a non-magnetic fraction of material contains some valuable components that can be magnetic separation [13]. used in various industries. In the initial period of plant operation (since 1955) In Russia, mining-induced waste occupies more than iron ore tailings were stored in the Southern Bay of 300 thousand hectares of lands. -

Phase Transition of Electrooxidized Fe3o4 to Γ and Α-Fe2o3 Nanoparticles Using Sintering Treatment I
Vol. 125 (2014) ACTA PHYSICA POLONICA A No. 5 Phase Transition of Electrooxidized Fe3O4 to γ and α-Fe2O3 Nanoparticles Using Sintering Treatment I. Kazeminezhad∗ and S. Mosivand Physics Department, Faculty of Science, Shahid Chamran University, Ahvaz, Iran (Received June 4, 2013; in nal form January 1, 2014) In this work, electrosynthesis of Fe3O4 nanoparticles was carried out potentiostatically in an aqueous solution of C4H12NCl which acts as supporting electrolyte and electrostatic stabilizer. γ-Fe2O3 nanoparticles were synthesized by controlling oxidation of the electrooxidized Fe3O4 nanoparticles at dierent temperature. Finally the phase transition to α-Fe2O3 nanoparticles was performed at high temperatures using sintering treatment. The synthesized particles were characterized using X-ray diraction, Fourier transformation, infrared scanning electron microscopy with energy dispersive X-ray analysis, and vibrating sample magnetometry. Based on the X-ray diraction results, ◦ ◦ the transition from Fe3O4 to cubic and tetragonal γ-Fe2O3 was performed at 200 C and 650 C, respectively. Furthermore, phase transition from metastable γ-Fe2O3 to stable α-Fe2O3 with rhombohedral crystal structure was ◦ approved at 800 C. The existence of the stabilizer molecules at the surface of Fe3O4 nanoparticles was conrmed by Fourier transformation infrared spectroscopy. According to scanning electron microscopy images, the average particles size was observed around 50 nm for electrooxidized Fe3O4 and γ-Fe2O3 nanoparticles prepared at sintering temperature lower than 900 ◦C, however by raising sintering temperature above 900 ◦C the mean particles size increases. Energy dispersive X-ray point analysis revealed that the nanoparticles are almost pure and composed of Fe and O elements. According to the vibrating sample magnetometry results, saturation magnetization, coercivity eld, and remnant magnetization decrease by phase transition from Fe3O4 to Fe2O3. -

Banded Iron Formations
Banded Iron Formations Cover Slide 1 What are Banded Iron Formations (BIFs)? • Large sedimentary structures Kalmina gorge banded iron (Gypsy Denise 2013, Creative Commons) BIFs were deposited in shallow marine troughs or basins. Deposits are tens of km long, several km wide and 150 – 600 m thick. Photo is of Kalmina gorge in the Pilbara (Karijini National Park, Hamersley Ranges) 2 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock Iron rich bands: hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). Iron poor bands: chert (fine‐grained quartz) and low iron oxide levels Rock sample from a BIF (Woudloper 2009, Creative Commons 1.0) Iron rich bands are composed of hematitie (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). The iron poor bands contain chert (fine‐grained quartz) with lesser amounts of iron oxide. 3 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock • Archaean and Proterozoic in age BIF formation through time (KG Budge 2020, public domain) BIFs were deposited for 2 billion years during the Archaean and Proterozoic. There was another short time of deposition during a Snowball Earth event. 4 Why are BIFs important? • Iron ore exports are Australia’s top earner, worth $61 billion in 2017‐2018 • Iron ore comes from enriched BIF deposits Rio Tinto iron ore shiploader in the Pilbara (C Hargrave, CSIRO Science Image) Australia is consistently the leading iron ore exporter in the world. We have large deposits where the iron‐poor chert bands have been leached away, leaving 40%‐60% iron. -

Smelting Iron from Laterite: Technical Possibility Or Ethnographic Aberration?
Smelting Iron from Laterite: Technical Possibility or Ethnographic Aberration? T. O. PRYCE AND S. NATAPINTU introduction Laterites deposits (orlateriticsoilsastheyarealsocalled)arefrequently reported in Southeast Asia, and are ethnographically attested to have been used for the smelting of iron in the region (Abendanon 1917 in Bronson 1992:73; Bronson and Charoenwongsa 1986), as well as in Africa (Gordon and Killick 1993; Miller and Van Der Merwe 1994). The present authors do not dispute this evidence; we merely wish to counsel cautioninitsextrapolation.Modifyingour understanding of a population’s potential to locally produce their own iron has immediate ramifications for how we reconstruct ancient metal distribution net- works, and the social exchanges that have facilitated them since iron’s generally agreed appearance in Southeast Asian archaeological contexts during the mid-first millennium b.c. (e.g., Bellwood 2007:268; Higham 1989:190). We present this paper as a wholly constructive critique of what appears to be a prevailingperspectiveonpre-modernSoutheastAsianironmetallurgy.Wehave tried to avoid technical language and jargon wherever possible, as our aim is to motivate scholars working within the regiontogivefurtherconsiderationtoiron as a metal, as a technology, and as a socially significant medium (e.g., Appadurai 1998; Binsbergen 2005; Gosden and Marshall 1999). When writing a critique it is of course necessary to cite researchers with whom one disagrees, and we have done this with full acknowledgment that in modern archaeology no one person can encompass the entire knowledge spectrum of the discipline.1 The archaeome- tallurgy of iron is probably on the periphery of most of our colleagues’ interests, but sometimes, within the technical, lies the pivotal, and in sharing some of our insights we hope to illuminate issues of benefit to all researchers in Metal Age Southeast Asia. -
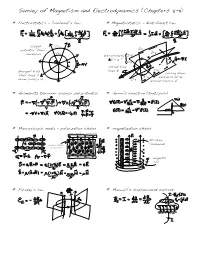
Survey of Magnetism and Electrodynamics (Chapters 5-11)
Survey of Magnetism and Electrodynamics (Chapters 5-11) * Electrostatics - Coulomb’s law * Magnetostatics - Biot-Savart law closed V=1 potential ”flow “ I=1 V=2 I=2 surfaces discontinuity I=0 I = 6 ! I=6 closed flux divergent field lines B I=3 curling flow ”flux “lines D I=4 surfaces of H from source Q I=5 around source I * Helmholtz theorem: source and potential * Green ‘s function (tent/pole) * Macroscopic media - polarization chains * magnetization chains free charge in conductor M-chain bound charge (solenoid) in dielectric magnetic coil * Faraday’s law * Maxwell’s displacement current * Three electrical devices, each the ratio of flux / flow CAPACITOR RESISTOR INDUCTOR * Electrodynamics equations Lorentz force Continuity Maxwell electric, magnetic fields Constitution Potentials (wave equation) Gauge transform * Conserved currents desity flux energy momentum * Electromagnetic waves - Fresnell’s coefficients - skin depth - dipole radiation Section 5.1.1 – Magnetic Fields * the magnetic force was known in antiquity, but was more difficult to quantify ~ predominant effect in nature involves magnetization, not electric currents ~ no magnetic (point) charge (monopole); 1-d currents instead of 0-d charges ~ static electricity was produced in the lab long before steady currents * History: http://maxwell.byu.edu/~spencerr/phys442/node4.html 600 BC Thales of Miletus discovers lodestone’s atraction to iron 1200 AD Chinese use lodestone compass for navigation 1259 AD Petrus Peregrinus (Italy) discovers the same thing 1600 AD William Gilbert -

Spinel Group Minerals in Metamorphosed Ultramafic Rocks from Río De Las Tunas Belt, Central Andes, Argentina
Geologica Acta, Vol.11, Nº 2, June 2013, 133-148 DOI: 10.1344/105.000001836 Available online at www.geologica-acta.com Spinel group minerals in metamorphosed ultramafic rocks from Río de Las Tunas belt, Central Andes, Argentina 1 1 2 M.F. GARGIULO E.A. BJERG A. MOGESSIE 1 INGEOSUR (Universidad Nacional del Sur – CONICET) San Juan 670, B8000ICN Bahía Blanca, Argentina Gargiulo E-mail: [email protected]; [email protected] Bjerg E-mail: [email protected] 2 Institut für Erdwissenschaften, Bereich Mineralogie und Petrologie, Karl-Franzens Universität Graz Universitätsplatz 2, 8010 Graz, Austria E-mail: [email protected] ABS TRACT In the Río de Las Tunas belt, Central Andes of Argentina, spinel group minerals occur in metaperidotites and in reaction zones developed at the boundary between metaperidotite bodies and their country-rocks. They comprise two types: i) Reddish-brown crystals with compositional zonation characterized by a ferritchromite core surrounded by an inner rim of Cr-magnetite and an outer rim of almost pure magnetite. ii) Green crystals chemically homogeneous with spinel (s.s.) and/or pleonaste compositions. The mineral paragenesis Fo+Srp+Cln+Tr+Fe-Chr and Fo+Cln+Tr+Tlc±Ath+Fe-Chr observed in the samples indicate lower and middle grade amphibolite facies metamorphic conditions. Nonetheless, the paragenesis (green)Spl+En+Fo±Di indicates that granulite facies conditions were also reached at a few localities. Cr-magnetite and magnetite rims in zoned reddish-brown crystals and magnetite rims around green-spinel/pleonaste grains are attributed to a later serpentinization process during retrograde metamorphism. -

Magnetic Rocks © Learning A–Z Learning A–Z J Written by Katherine Follett Lexile 470L
FOCUS Book Magnetic Make a magnet! Get a strong magnet and a thin metal bolt. Rub the bolt Rocks across the magnet in one direction fifty times. Pick up a staple with the bolt. You made a magnet! Design a test for your magnet. What can it pick up? What else can you test? Now make another magnet with a new bolt. Change one thing about how you make the magnet. Then repeat your test. How did the results change? Beyond the Book Go to two places with sand. Find out which kind of sand has the most magnetite. Metal and Magnets Magnetic Have you ever played with magnets? Maybe there are magnets on your Rocks refrigerator. Some toys also have magnets in them. A magnet is a special piece of metal. It pulls on, or attracts, other pieces FOCUS Question of metal. What kinds of natural materials stick to magnets? But where do magnets come from? Cause and Effect Photo Credits: Front cover: © Joel Arem/Science Source/Getty Images; page 2: © Vanessa Davies/DK Images; page 3: © Art Directors & TRIP/Alamy Stock Photo; page 4: © Richard Hutchings/Science Source; page 5 (top): © Charles O’Rear/Corbis/VCG/Corbis Documentary/Getty Images; page 5 (bottom): © David Lee/ Alamy Stock Photo; page 6 (left): Sarah Cebulski/© Learning A–Z; page 6 (right): © Alexandru Dobrea/ Hemera/Thinkstock; page 7 (top): © Visuals Unlimited/Getty Images; page 7 (bottom): © Liu Liqun/Corbis Documentary/Getty Images; page 9: © The Natural History Museum/Alamy Stock Photo Illustration Credits: Page 8: Signe Nordin/© Learning A–Z; border (rock): Nora Voutas/© Learning A–Z; (clip): Casey Jones/ © Learning A–Z Reading Levels Magnetic Rocks © Learning A–Z Learning A–Z J Written by Katherine Follett Lexile 470L Correlations All rights reserved. -

ITP Mining: Energy and Environmental Profile of the U.S. Mining Industry: Chapter 4: Iron
Energy and Environmental Profile of the U.S. Mining Industry 4 Iron The chemical element iron is the fourth most common element in the Earth's crust and the second most abundant metal. About five percent of the Earth's crust is composed of iron. The metal is chemically active and is found in nature combined with other elements in rocks and soils. In its natural state, iron is chemically bonded with oxygen, water, carbon dioxide, or sulfur in a variety of minerals. Forms of Iron Minerals, Ores, and Rocks Iron occurs mainly in iron-oxide ores. Some ores are a mixture of minerals rich in iron. Other iron ores are less rich and have a large number of impurities. The most important iron ore- forming minerals are: • Magnetite - Magnetite (Fe3O4) forms magnetic black iron ore. There are large deposits of magnetite in Russia and Sweden. • Hematite - Hematite (Fe2O3) is a red iron ore. Hematite occurs in almost all forms, from solid rock to loose earth. It is the most plentiful iron ore and occurs in large quantities throughout the world. • Goethite - Goethite (Fe2O3.H2O), a brown ore, contains iron. • Limonite - Limonite (Fe2O3.H2O) is a yellow-brown iron ore. Limonite is a collective term for impure goethite and a mixture of hydrated iron oxides. Iron 4-1 Energy and Environmental Profile of the U.S. Mining Industry Taconite contains low-grade iron in fine specks and bands. It is an extremely hard and flinty - containing about 25 - 30 percent iron. The iron in taconite occurs principally as magnetite and hematite and finely dispersed with silica in sedimentary deposits.