Emerging Issues in Infective Endocarditis Beverley C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter Xi the Circulatory System and Blood
CHAPTER XI THE CIRCULATORY SYSTEM AND BLOOD Page General characterlstlcs______ __ __ _ __ __ __ __ __ __ _ 239 of these organs are independent of the beating of The pericardium ___ __ __ __ 239 the principal heart, and their primary function is The heart. _____ __ __ 240 Physiology of the heart.______________________________________________ 242 to oscillate the blood within the pallial sinuses. Automatism of heart beat. _ 242 The pacemaker system_ 245 THE PERICARDIUM Methods of study of heart beat_____________________________________ 247 Frequency of beat___ __ __ _ 248 Extracardlac regulatlon____ __ __ _ 250 The heart is located in the pericardium, a thin Effects of mineral salts and drugs___________________________________ 251 Blood vessels_ __ ___ _ 253 walled chamber between the visceral mass and the The arterial system______ __ __ ___ __ __ __ __ __ 253 adductor muscle (fig. 71). In a live oyster the The venous system_________________________________________________ 254 location of the heart is indicated by the throbbing The accessory heart._____________ 258 The blood______ __ __ __ __ __ __ __ 259 of the wall of the pericardium on the left side. Color of blood_ __ __ 261 Here the pericardium wall lies directly under the The hyaline cells___________________________________________________ 261 The granular cells .______________________________________ 262 shell. On the right side the promyal chamber Specific gravity of blood____________________________________________ 265 extends down over the heart region and the mantle Serology ___ __________ __________________ ____ __ ______________________ 265 Bibliography __ __ __ __ __ __ __ 266 separates the pericardium wall from the shell. The cavity in which the heart is lodged is slightly A heart, arteries, veins, and open sinuses form asymmetrical; on the right side it extends farther the circulatory system of oysters and other bi along the anterior part of the adductor muscle valves. -

CASE REPORTS Supraventricular Tachycardia As the Initial
http://crim.sciedupress.com Case Reports in Internal Medicine, 2015, Vol. 2, No. 3 CASE REPORTS Supraventricular tachycardia as the initial presentation of bacterial infective endocarditis: A rare case report Wei-Tsung Wu1, Hung-Ling Huang2, Ho-Ming Su1, 3, Tsung-Hsien Lin1, 3, Kun-Tai Lee1, 3, Sheng-Hsiung Sheu1, 3, Po-Chao Hsu1, 3 1. Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, ROC. 2. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, ROC. 3. Department of Internal Medicine, Faculty of Medicine, School of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, ROC Correspondence: Po-Chao Hsu. Address: Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, 100 Tzyou 1st Road, Kaohsiung. 80708, Taiwan, ROC. Email: [email protected] Received: May 19, 2015 Accepted: June 15, 2015 Online Published: June 17, 2015 DOI: 10.5430/crim.v2n3p26 URL: http://dx.doi.org/10.5430/crim.v2n3p26 Abstract Infective endocarditis (IE) is an infection of the heart valves or the heart’s inner lining. The clinical symptoms of infectious endocarditis vary considerably, some are subtle and non-specific, which make the diagnosis difficult or the signs misleading. In some cases cardiac symptoms are associated with intra cardiac extension of the infection, including murmur, conduction blocks and congestive heart failure. However, there was no literature description of supraventricular tachycardia (SVT) as the initial presentation of IE. Herein we report a case of bacterial IE with SVT as the initial presentation of disease, which finally leaded to catastrophic outcome. -
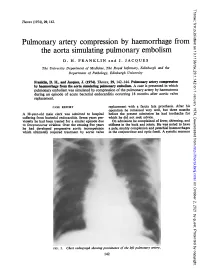
The Aorta Simulating Pulmonary Embolism D
Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. Downloaded from Thorax (1974), 29, 142. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism D. H. FRANKLIN and J. JACQUES The University Department of Medicine, The Royal Infirmary, Edinburgh and the Department of Pathology, Edinburgh University Franklin, D. H., and Jacques, J. (1974). Thorax, 29, 142-144. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism. A case is presented in which pulmonary embolism was simulated by compression of the pulmonary artery by haematoma during an episode of acute bacterial endocarditis occurring 18 months after aortic valve replacement. CASE REPORT replacement with a fascia lata prosthesis. After his operation he remained very well, but three months A 38-year-old male clerk was admitted to hospital before the present admission he had toothache for suffering from bacterial efndocarditis. Seven years pre- which he did not seek advice. viomly he had been treated for a similar episode due On admission he complained of fever, shivering, and to Streptococcus viridans. Over the ensuing five years stiffness in the back and joints. He was noted to have he had developed progressive aortic incompetence a pale, muddy complexion and petechial haemorrhages which ultimately required treatment by aortic valve in the conjunctivae and optic fundi. A systolic murmur http://thorax.bmj.com/ on October 2, 2021 by guest. Protected copyright. FIG. 1. Chesvt radiograph showing prominence of the left pulmonary artery. 142 Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. -

Transesophageal Echocardiographic Detection of Cardiac Embolic Source in Cor Triatriatum Complicated by Aortic Saddle Emboli
294 N. Cohen et al.: Brucellu endocarditis 4. Delvecchio G, Fracassetti 0, Lorenzi N: Brucellu endocarditis.fnt 15. Farid Z, Trabolsi B: Successful treatment of IWO cases of Br~rlltr J Curdiol1991 ;33:328-329 endocarditiswith rifampicin. BrMedJ 1985;29I : 1 10 5. Jeroudi MO, Halim MA, Harder EJ, Al-Sibai MB, Ziady G, 16. Al-Harthi SS: The morbidity and mortality pattern ofB~.~ccd/~ren- Mercer EN: Brucellu endocarditis.Br Heart J 1987;58:279-283 docarditis. Int J CurdiolI989:25:321-324 6. Fernandez-Guerrero ML: Zoonotic endocarditis. lnfect Dis Clin 17. Quinn RW, Brown JW Bacterial endocarditis. Arch hirm Md North Am 3993;7:135-1 52 1954;94:679684 7. Pazderka E, Jones JW: BruceNu abortus endocarditis: Successful 18. Micozzi A, Venditti M, Gentile G, Alessandii N, Santero M, Mar- treatment of an infected aortic valve. Arch Intern Med 1982;142: tino P: Successful treatment of Bvucelkc nic~/itrnsi.tendocarditis 1567- I568 with pefloxacin. EuvJ Clin Microhiollnjiw Di.v 1990;9:44W? 8. Valliattu J, Shuhaiber H, Kiwan Y, Araj G, Chugh T Brucellu en- 19. Al Mudallal DS, Mousa ARM, Marafie AA: Apyrcxic H~~rrc~c~lltr docarditis: Report of one case and review of the literature. J Cur- melitensis aortic valve endocarditis. Trop Gc~pMeti 1989i-l I : diovusc Surg 1989;30:782-785 372-376 9. Al-Kasab S, AI-Faghin MR, Al-Yousef S, Ali Khan MA, Ribeiro 20. Peery TM, Belter LF: Brucellosis and heart disease. Fatal hrucel- PA, Nazzal S, Al-Zaibag M: Brucellu infective endocarditis: Suc- losis: A review of the literature and repon of ncw cahes. -

Premature Obliteration of the Foramen Ovale by G
PREMATURE OBLITERATION OF THE FORAMEN OVALE BY G. AUSTIN GRESHAM From the Department of Pathology, University of Cambridge Received August 16, 1955 Obliteration of the foramen ovale occurring during intrauterine life is a rare condition. It throws some light on the mechanism of development of endocardial fibroelastosis and also upon the factors concerned in determining the size of the aorta and of the cardiac chambers. CASE HISTORY The patient was the second child of a mother (aet. 21) whose previous obstetric history was normal. Apart from two periods of rather rapid gain of weight for which no cause could be found, the pregnancy was uneventful. The child was eleven days postmature; labour was induced with an enema and lasted forty-five minutes. The infant cried lustily but was cyanosed: the heart was clinically normal. Abnormalities were present in all four limbs. The left radius and ulna were absent and rudimentary digits were present on the skin over the distal end of the limb. Terminal phalanges were absent in the fingers of the right hand, and four toes were present on each foot with a rudimentary fifth digit on the left foot. Cyanosis and dyspniea became more intense and the child died three hours after birth despite the use of oxygen. NECROPSY The body was that of a full-term male infant (weight 3430 g.). The limbs were abnormal as previously described. The lips were blue-black in colour. On the septal wall of the right atrium a hemispherical grey-white area (10x 12 x 4 mm. deep) with a central dimple filled in the usual site of the fossa ovalis (Fig. -

Prevention of Infective Endocarditis: Revised Guidelines from the American Heart Association and the Implications for Dentists
Clinical P RACTIC E Prevention of Infective Endocarditis: Revised Guidelines from the American Heart Association and the Implications for Dentists Contact Author David K. Lam, DDS; Ahmed Jan, DDS; George K.B. Sándor, MD, DDS, PhD, FRCD(C), FRCSC, FACS; Dr. Sándor Email: george.sandor@ Cameron M.L. Clokie, DDS, PhD, FRCD(C) utoronto.ca ABSTRACT Infective endocarditis is a rare but life-threatening microbial infection of the heart valves or endocardium, most often related to congenital or acquired cardiac defects. The American Heart Association (AHA) recently updated its recommendations on prophylaxis during dental procedures. The revisions will have a profound impact on both the patient and the dental practitioner. The purpose of this paper is to review the pathogenesis and clinical presentation of infective endocarditis and discuss the 2007 AHA guidelines and their implications for dentists. For citation purposes, the electronic version is the definitive version of this article: www.cda-adc.ca/jcda/vol-74/issue-5/449.html nfective endocarditis is an uncommon but American Dental Association, the Infectious potentially life-threatening microbial in- Diseases Society of America and the American Ifection of the heart valves or endocardium, Academy of Pediatrics, to review input from most often related to congenital or acquired national and international experts on the cardiac defects. High morbidity and mortality disease. The recommendations of this group rates remain associated with the infection culminated in the 2007 AHA guidelines on despite advances in diagnosis, antimicrobial prophylaxis for infective endocarditis, which therapy, surgical techniques and manage- will have a profound impact on both patients ment of associated complications. -

The Cardiovascular System
11 The Cardiovascular System WHAT The cardiovascular system delivers oxygen and HOW nutrients to the body tissues The heart pumps and carries away wastes blood throughout the body such as carbon dioxide in blood vessels. Blood flow via blood. requires both the pumping action of the heart and changes in blood pressure. WHY If the cardiovascular system cannot perform its functions, wastes build up in tissues. INSTRUCTORS Body organs fail to function properly, New Building Vocabulary and then, once oxygen becomes Coaching Activities for this depleted, they will die. chapter are assignable in hen most people hear the term cardio- only with the interstitial fluid in their immediate Wvascular system, they immediately think vicinity. Thus, some means of changing and of the heart. We have all felt our own “refreshing” these fluids is necessary to renew the heart “pound” from time to time when we are ner- nutrients and prevent pollution caused by vous. The crucial importance of the heart has been the buildup of wastes. Like a bustling factory, the recognized for ages. However, the cardiovascular body must have a transportation system to carry system is much more than just the heart, and its various “cargoes” back and forth. Instead of from a scientific and medical standpoint, it is roads, railway tracks, and subways, the body’s important to understand why this system is so vital delivery routes are its hollow blood vessels. to life. Most simply stated, the major function of the Night and day, minute after minute, our tril- cardiovascular system is transportation. Using lions of cells take up nutrients and excrete wastes. -

The Surgical Treatment of Constrictive Fibrous Endocarditis
The Surgical Treatment of Constrictive Fibrous Endocarditis CH. DUBOST, P. MAURICE, A. GERBAUX, E. BERTRAND, R. RULLIERE, F. VIAL, A. BARRILLON, C. PRIGENT, A. CARPENTIER, R. SOYER Constrictive fibrous endocarditis is a pathological entity described From the Department of Cardiovascular Surgery, by Lo6ffler in 1936. Its etiology is unknown. The clinical course Broussais Hospital, Paris, France is characterized by an evolution towards cardiac insufficiency leading rapidly to a fatal outcome. Modern paraclinical investi- gations are necessary to assess the diagnostic. Cardiac catheteriza- tion brings the proof of adiastole and angiocardiography re- Endocardiectomy was first performed by us in 19715-7 veals the shape of amputation of the ventricle with auriculo- and the patient was improved. Since then, four more cases ventricular regurgitation. The operative procedure consists of have been done, either right or left sided or combined resection of the ventricular fibrosis including the valves and endocardiectomy and there is no doubt that a better auriculo-ventricular valve replacement 'by ;a prosthetic valve. The disease affects both Caucasians and Negros. Our experience knowledge of the clinical aspects of the disease will includes 5 cases. The indications for operation and their results increase the number of cases referred for treatment. are discussed. Pathology C ONSTRICTIVE FIBROUS ENDOCARDITIS, first described The development of fibrous endocarditis is similar in by Loeffler6 in 1936, consists of endocardial fibrosis all cases: it involves the filling chambers of one or several millimeters in thickness which is potentially both ventricles including the papillary muscles and constrictive and limited to the ventricular cavities. chordae tendinae of the mitral and tricuspid valves The clinical course is characterized by an evolution which themselves are included in the process in the towards cardiac insufficiency which may be predomi- majority of cases.8 nantly right or left sided leading rapidly to a fatal outcome. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5]. -

Inflammation and Your Heart: Endocarditis, Pericarditis and Myocarditis
Inflammation and your heart: Endocarditis, pericarditis and myocarditis Types of inflammation Myocarditis When you see the letters ‘itis’ at the end of a What causes myocarditis? Will I need treatment? word, it means inflammation. Myocarditis is inflammation of the myocardium Myocarditis is often mild and goes unnoticed, but – the heart muscle. It is usually caused by a viral, you may need to take medicines to relieve your Myocarditis, pericarditis and bacterial or fungal infection. Sometimes the cause is symptoms such as non-steroidal anti-inflammatories endocarditis refer to inflammation unknown – or ‘idiopathic’. and sometimes antibiotics. around or in the heart. If the myocarditis it is causing a problem with What are the symptoms? how well your heart pumps, you may develop the • Myocarditis – inflammation of the myocardium The symptoms of myocarditis usually include a (the heart muscle) symptoms of heart failure which you will need to pain or tightness in your chest which can spread to take several different types of medicines for. In very • Pericarditis – inflammation of the pericardium other parts of your body, shortness of breath and extreme cases where there is severe damage to the (the sac which surrounds tiredness. You may also have flu like symptoms, such heart you may be considered for a heart transplant. the heart) as a high temperature, feeling tired, headaches and aching muscles and joints. • Endocarditis – inflammation of the Inflammation of the heart often causes chest pain, endocardium (the inner lining of the heart) What tests will I need? and you may feel like you are having a heart attack. If you have not been diagnosed with one of You may need to have an electrocardiogram (ECG), these conditions and you have chest pain, or any echocardiogram (a scan of your heart similar to an of the symptoms we describe below, call 999 ultrasound) and various blood tests. -

Structure of the Heart 2121
8941d_c21.qxd 8/13/03 2:19 PM Page 249 mac 106 mac 106:Desktop Folder:211_sks: EXERCISE STRUCTURE OF THE HEART 2121 OBJECTIVES MATERIALS After completing this exercise, you should • human heart model be able to: • human torso or chart showing the pulmonary and • Describe the location and coverings of the heart systemic circulations and the three layers of the heart wall • colored pencils • Identify major features of cardiac muscle tissue on • preserved sheep heart (or other mammal heart) a prepared microscope slide • dissecting instruments, trays, disposable gloves, • Identify the major heart structures on models or 5-inch blade knife charts • compound microscope with cardiac muscle slide • Describe the flow of a drop of blood through the (for demonstration) pulmonary and systemic circulations, listing the vessels, chambers, and valves • Describe the changes that take place in the heart after birth • Identify the selected heart structures on a dissected sheep heart our heart beats without external stimulation and rests A. COVERINGS only between heart beats. The heart is a small double Ypump that simultaneously pumps blood to and from AND LAYERS body cells through the systemic circulation and to and from OF THE HEART the lungs through the pulmonary circulation. The heart is about the size of a fist and lies in the thoracic cavity. The base of the heart is the wide superior portion of the heart from which the great vessels emerge, and the apex of the heart is the inferior end pointing to the left. The heart is tilted at an angle so that its inferior surface lies against the diaphragm with two-thirds of the heart to the left side of the sternum. -

Infective Endocarditis: a Focus on Oral Microbiota
microorganisms Review Infective Endocarditis: A Focus on Oral Microbiota Carmela Del Giudice 1 , Emanuele Vaia 1 , Daniela Liccardo 2, Federica Marzano 3, Alessandra Valletta 1, Gianrico Spagnuolo 1,4 , Nicola Ferrara 2,5, Carlo Rengo 6 , Alessandro Cannavo 2,* and Giuseppe Rengo 2,5 1 Department of Neurosciences, Reproductive and Odontostomatological Sciences, Federico II University of Naples, 80131 Naples, Italy; [email protected] (C.D.G.); [email protected] (E.V.); [email protected] (A.V.); [email protected] (G.S.) 2 Department of Translational Medical Sciences, Medicine Federico II University of Naples, 80131 Naples, Italy; [email protected] (D.L.); [email protected] (N.F.); [email protected] (G.R.) 3 Department of Advanced Biomedical Sciences, University of Naples Federico II, 80131 Naples, Italy; [email protected] 4 Institute of Dentistry, I. M. Sechenov First Moscow State Medical University, 119435 Moscow, Russia 5 Istituti Clinici Scientifici ICS-Maugeri, 82037 Telese Terme, Italy 6 Department of Prosthodontics and Dental Materials, School of Dental Medicine, University of Siena, 53100 Siena, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0817463677 Abstract: Infective endocarditis (IE) is an inflammatory disease usually caused by bacteria entering the bloodstream and settling in the heart lining valves or blood vessels. Despite modern antimicrobial and surgical treatments, IE continues to cause substantial morbidity and mortality. Thus, primary Citation: Del Giudice, C.; Vaia, E.; prevention and enhanced diagnosis remain the most important strategies to fight this disease. In Liccardo, D.; Marzano, F.; Valletta, A.; this regard, it is worth noting that for over 50 years, oral microbiota has been considered one of the Spagnuolo, G.; Ferrara, N.; Rengo, C.; significant risk factors for IE.