Post-Traumatic Epilepsy: Research at the VA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Altered Protein Profiles During Epileptogenesis in the Pilocarpine
ORIGINAL RESEARCH published: 28 May 2021 doi: 10.3389/fneur.2021.654606 Altered Protein Profiles During Epileptogenesis in the Pilocarpine Mouse Model of Temporal Lobe Epilepsy Md. Mahiuddin Ahmed 1, Andrew J. Carrel 2, Yasmin Cruz Del Angel 2, Jessica Carlsen 2, Ajay X. Thomas 2,3,4, Marco I. González 2, Katheleen J. Gardiner 5† and Amy Brooks-Kayal 2,6,7,8*† 1 Department of Neurology, University of Colorado Alzheimer’s and Cognition Center, Linda Crnic Institute for Down Syndrome, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2 Division of Neurology and Translational Epilepsy Research Program, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO, United States, 3 Section of Neurology and Developmental Neuroscience, Department of Pediatrics, Baylor College of Medicine, Houston, TX, United States, 4 Section of Child Neurology, Texas Children’s Hospital, Houston, TX, United States, Edited by: 5 Department of Pediatrics, Linda Crnic Institute for Down Syndrome, University of Colorado Anschutz Medical Campus, Kjell Heuser, Aurora, CO, United States, 6 Department of Pharmaceutical Sciences, Skaggs School of Pharmacy and Pharmaceutical Oslo University Hospital, Norway Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 7 Children’s Hospital Colorado, Reviewed by: Aurora, CO, United States, 8 Department of Neurology, University of California Davis School of Medicine, Sacramento, CA, Victor Rodrigues Santos, United States Federal University of Minas Gerais, Brazil Divya Vohora, Epilepsy is characterized by recurrent, spontaneous seizures and is a major contributor Jamia Hamdard University, India to the global burden of neurological disease. Although epilepsy can result from a variety *Correspondence: of brain insults, in many cases the cause is unknown and, in a significant proportion Amy Brooks-Kayal of cases, seizures cannot be controlled by available treatments. -

Type of the Paper (Article
Supplementary Material A Proteomics Study on the Mechanism of Nutmeg-induced Hepatotoxicity Wei Xia 1, †, Zhipeng Cao 1, †, Xiaoyu Zhang 1 and Lina Gao 1,* 1 School of Forensic Medicine, China Medical University, Shenyang 110122, P. R. China; lessen- [email protected] (W.X.); [email protected] (Z.C.); [email protected] (X.Z.) † The authors contributed equally to this work. * Correspondence: [email protected] Figure S1. Table S1. Peptide fraction separation liquid chromatography elution gradient table. Time (min) Flow rate (mL/min) Mobile phase A (%) Mobile phase B (%) 0 1 97 3 10 1 95 5 30 1 80 20 48 1 60 40 50 1 50 50 53 1 30 70 54 1 0 100 1 Table 2. Liquid chromatography elution gradient table. Time (min) Flow rate (nL/min) Mobile phase A (%) Mobile phase B (%) 0 600 94 6 2 600 83 17 82 600 60 40 84 600 50 50 85 600 45 55 90 600 0 100 Table S3. The analysis parameter of Proteome Discoverer 2.2. Item Value Type of Quantification Reporter Quantification (TMT) Enzyme Trypsin Max.Missed Cleavage Sites 2 Precursor Mass Tolerance 10 ppm Fragment Mass Tolerance 0.02 Da Dynamic Modification Oxidation/+15.995 Da (M) and TMT /+229.163 Da (K,Y) N-Terminal Modification Acetyl/+42.011 Da (N-Terminal) and TMT /+229.163 Da (N-Terminal) Static Modification Carbamidomethyl/+57.021 Da (C) 2 Table S4. The DEPs between the low-dose group and the control group. Protein Gene Fold Change P value Trend mRNA H2-K1 0.380 0.010 down Glutamine synthetase 0.426 0.022 down Annexin Anxa6 0.447 0.032 down mRNA H2-D1 0.467 0.002 down Ribokinase Rbks 0.487 0.000 -

Compositions and Methods for Selective Delivery of Oligonucleotide Molecules to Specific Neuron Types
(19) TZZ ¥Z_T (11) EP 2 380 595 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 26.10.2011 Bulletin 2011/43 A61K 47/48 (2006.01) C12N 15/11 (2006.01) A61P 25/00 (2006.01) A61K 49/00 (2006.01) (2006.01) (21) Application number: 10382087.4 A61K 51/00 (22) Date of filing: 19.04.2010 (84) Designated Contracting States: • Alvarado Urbina, Gabriel AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Nepean Ontario K2G 4Z1 (CA) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Bortolozzi Biassoni, Analia Alejandra PT RO SE SI SK SM TR E-08036, Barcelona (ES) Designated Extension States: • Artigas Perez, Francesc AL BA ME RS E-08036, Barcelona (ES) • Vila Bover, Miquel (71) Applicant: Nlife Therapeutics S.L. 15006 La Coruna (ES) E-08035, Barcelona (ES) (72) Inventors: (74) Representative: ABG Patentes, S.L. • Montefeltro, Andrés Pablo Avenida de Burgos 16D E-08014, Barcelon (ES) Edificio Euromor 28036 Madrid (ES) (54) Compositions and methods for selective delivery of oligonucleotide molecules to specific neuron types (57) The invention provides a conjugate comprising nucleuc acid toi cell of interests and thus, for the treat- (i) a nucleic acid which is complementary to a target nu- ment of diseases which require a down-regulation of the cleic acid sequence and which expression prevents or protein encoded by the target nucleic acid as well as for reduces expression of the target nucleic acid and (ii) a the delivery of contrast agents to the cells for diagnostic selectivity agent which is capable of binding with high purposes. -

Transport of Pregabalin Via L-Type Amino Acid Transporter 1 (SLC7A5) in Human Brain Capillary Endothelial Cell Line
Pharm Res (2018) 35: 246 https://doi.org/10.1007/s11095-018-2532-0 RESEARCH PAPER Transport of Pregabalin Via L-Type Amino Acid Transporter 1 (SLC7A5) in Human Brain Capillary Endothelial Cell Line Yu Takahashi1 & Tomohiro Nishimura 1 & Kei Higuchi 2 & Saki Noguchi 1 & Yuma Tega 2 & Toshiki Kurosawa2 & Yo s h i h a r u D e g u c h i 2 & Masatoshi Tomi1 Received: 14 July 2018 /Accepted: 21 October 2018 /Published online: 29 October 2018 # The Author(s) 2018 ABSTRACT Conclusions Our results indicate that LAT1, but not LAT2, Purpose The anti-epileptic drug pregabalin crosses the recognizes pregabalin as a substrate. It is suggested that LAT1 blood-brain barrier (BBB) in spite of its low lipophilicity. mediates pregabalin transport at the BBB. This study was performed to determine whether L-type amino acid transporters (LAT1/SLC7A5 and LAT2/SLC7A8) con- KEYWORDS anti-epileptic drug . blood-brain barrier . tribute to the uptake of pregabalin. L-type amino acid transporter . pregabalin Methods Pregabalin uptake by LATs-transfected HEK293 cells or hCMEC/D3 cells, an in vitro human BBB model, was measured by LC-MS/MS analysis. Expression of LAT1 mRNA in hCMEC/D3 cells was determined by quantitative ABBREVIATIONS RT-PCR analysis. BBB Blood-brain barrier Results Overexpression of LAT1, but not LAT2, in HEK293 BCH 2-Aminobicyclo-(2,2,1)-heptane-2-car- cells significantly increased the cellular uptake of pregabalin, boxylic acid GABA γ and the LAT1-mediated uptake was saturable with a Km of -Aminobutyric acid 0.288 mM. LAT1-mediated amino acid uptake was inhibited hCMEC/D3 cells Human immortalized brain endothelial specifically and almost completely in the presence of 1 mM cell line pregabalin. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Subsets Underlies IL-6 Signaling in Glial Β Differential
Differential TGF-β Signaling in Glial Subsets Underlies IL-6−Mediated Epileptogenesis in Mice This information is current as Nitzan Levy, Dan Z. Milikovsky, Gytis Baranauskas, of October 1, 2021. Ekaterina Vinogradov, Yaron David, Maya Ketzef, Shai Abutbul, Itai Weissberg, Lyn Kamintsky, Ilya Fleidervish, Alon Friedman and Alon Monsonego J Immunol 2015; 195:1713-1722; Prepublished online 1 July 2015; doi: 10.4049/jimmunol.1401446 Downloaded from http://www.jimmunol.org/content/195/4/1713 References This article cites 50 articles, 13 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/195/4/1713.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2015 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Differential TGF-b Signaling in Glial Subsets Underlies IL-6–Mediated Epileptogenesis in Mice Nitzan Levy,*,†,1 Dan Z. -

Post Traumatic Epilepsy : a Review of Scientific Evidence
Indian J Physiol Pharmacol 2006; 50 (1) : 7–16 REVIEW ARTICLE POST TRAUMATIC EPILEPSY : A REVIEW OF SCIENTIFIC EVIDENCE Y. K. GUPTA* AND MADHUR GUPTA Neuropharmacology Laboratory, Department of Pharmacology, All India Institute of Medical Sciences, New Delhi – 110 029 ( Received on July 15, 2005 ) Abstract : Post traumatic epilepsy is the development of recurrent seizures following head trauma and has a high clinical relevance. Several risk factors including some genetic factors increase the susceptibility of post traumatic epilepsy. The precise mechanisms of epileptogenesis in post-traumatic epilepsy are still poorly understood. Many structural, physiologic and biochemical changes in the brain may account for . epileptogenesis. The reactive oxygen species (ROS), especially OH and excitotoxicity are primarily involved. Antioxidants, like tocopherol, antiepileptic drug zonisamide, condensed tannins, melatonin, adenosine, trans-resveratrol, and some other agents have been proposed to prevent epileptogenic focus formation. The review also discusses various aspects of post traumatic epilepsy, mechanisms of epileptogenesis, and clinical implications. Key words : epilepsy trauma posttraumatic seizure oxidative stress INTRODUCTION recurrent epileptic seizures due to brain damage (2). It complicates the management Epilepsy is one of the most common of the head injuries patients by increasing neurological disorders affecting nearly 0.5 the intracranial pressure and altering percent of the world population (1). the level of unconsciousness. Among the Traumatic brain injury, which is a major long term complications of traumatic cause of morbidity and mortality world wide, brain injury, post traumatic epilepsy has been reported to be one of the major remains one of the most troubling and in risk factor for epileptic seizures. -

O'neill2020 Redacted.Pdf (7.617Mb)
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Functional Characterisation of Spontaneously Active GABAA Receptors in Rat Dentate Gyrus Granule Cells Nathanael O’Neill B.Medsc. (Hons) Doctor of Philosophy The University of Edinburgh 2020 ii Abstract GABAA receptors (GABAARs) are the principal inhibitory neurotransmitter receptors in the adult mammalian central nervous system. GABAARs mediate two forms of inhibition: fast, phasic conductance; and slow, tonic conductance. Tonic conductance arises due to the persistent activation of GABAARs. This persistent activation can occur by GABA-dependent or GABA- independent mechanisms. Low concentrations of ambient GABA activate high affinity GABAARs located outside the synapse – at peri-/extra-synaptic sites – to generate GABA-dependent tonic conductance. In contrast, GABA-independent tonic conductance is generated by GABAARs that activate spontaneously, in the absence of GABA, due to constitutive receptor gating. -

Reactive Astrocyte-Driven Epileptogenesis Is Induced by Microglia Initially Activated Following Status Epilepticus
Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus Fumikazu Sano, … , Masao Aihara, Schuichi Koizumi JCI Insight. 2021;6(9):e135391. https://doi.org/10.1172/jci.insight.135391. Research Article Neuroscience Graphical abstract Find the latest version: https://jci.me/135391/pdf RESEARCH ARTICLE Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus Fumikazu Sano,1,2,3 Eiji Shigetomi,1,3 Youichi Shinozaki,1,3 Haruka Tsuzukiyama,1 Kozo Saito,1,3,4 Katsuhiko Mikoshiba,5 Hiroshi Horiuchi,6 Dennis Lawrence Cheung,6 Junichi Nabekura,6 Kanji Sugita,2 Masao Aihara,2 and Schuichi Koizumi1,3 1Department of Neuropharmacology, Interdisciplinary Graduate School of Medicine, 2Department of Pediatrics, Faculty of Medicine, and 3Yamanashi GLIA Center, Interdisciplinary Graduate School of Medicine, University of Yamanashi, Yamanashi, Japan. 4Department of Neurology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan. 5Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University, Shanghai, China. 6Division of Homeostatic Development, National Institute for Physiological Sciences, Okazaki, Japan. Extensive activation of glial cells during a latent period has been well documented in various animal models of epilepsy. However, it remains unclear whether activated glial cells contribute to epileptogenesis, i.e., the chronically persistent process leading to epilepsy. Particularly, it is not clear whether interglial communication between different types of glial cells contributes to epileptogenesis, because past literature has mainly focused on one type of glial cell. Here, we show that temporally distinct activation profiles of microglia and astrocytes collaboratively contributed to epileptogenesis in a drug-induced status epilepticus model. -

Epileptogenesis Causes Long-Term Plasticity Changes in Calbindin D-28K in the Rat Pilocarpine Model of Acquired Epilepsy
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2005 Epileptogenesis Causes Long-Term Plasticity Changes in Calbindin D-28k in the Rat Pilocarpine Model of Acquired Epilepsy Anne Johnston Harrison Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Neurology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/855 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. EPILEPTOGENESIS CAUSES LONG-TERM PLASTICITY CHANGES IN EXPRESSION OF CALBINDIN D-28K IN THE RAT PILOCARPINE MODEL OF ACQUIRED EPILEPSY A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University Anne Elizabeth Johnston Harrison Bachelor of Science in Pharmacy Virginia Commonwealth University May 1996 Bachelor of Science in Psychology The College of William and Mary May 1993 Director: Robert J. DeLorenzo, M.D., Ph.D., M.P.H. Professor Department of Neurology Virginia Convnonwealth University Richmond, Virginia December 2005 DEDICATION To my husband, Steve, for all of his encouragement, love, and support. Te amo mejo. ACKNOWLEDGEMENTS I would like to take this opportunity to thank a number of people without whom I could not have completed this thesis. Each of you has made immeasurable contributions to my education and personal growth. First, I must credit my family for their constant support, love, and encouragement in all of my endeavors, and for emphasizing the significance of continued learning throughout life. -

Clinical Approach to Posttraumatic Epilepsy
57 Clinical Approach to Posttraumatic Epilepsy Vikram R. Rao, MD, PhD1 Karen L. Parko, MD1,2 1 Department of Neurology, University of California, Address for correspondence Vikram R. Rao, MD, PhD, University of San Francisco, California California, San Francisco, Epilepsy Center, 400 Parnassus Ave, 8th 2 Department of Neurology, San Francisco Veterans Affairs Medical Floor, San Francisco, CA 94143 (e-mail: [email protected]). Center, San Francisco, California Semin Neurol 2015;35:57–63. Abstract Traumatic brain injury (TBI) is one of the most common causes of acquired epilepsy, and posttraumatic epilepsy (PTE) results in significant somatic and psychosocial morbidity. The risk of developing PTE relates directly to TBI severity, but the latency to first seizure can be decades after the inciting trauma. Given this “silent period,” much work has focused on identification of molecular and radiographic biomarkers for risk stratification and on development of therapies to prevent epileptogenesis. Clinical management requires vigilant neurologic surveillance and recognition of the heterogeneous endo- Keywords phenotypes associated with PTE. Appropriate treatment of patients who have or are at ► traumatic brain injury risk for seizures varies as a function of time after TBI, and the clinician’s armamentarium ► epilepsy includes an ever-expanding diversity of pharmacological and surgical options. Most ► posttraumatic recently, neuromodulation with implantable devices has emerged as a promising epilepsy therapeutic strategy for some patients with refractory PTE. Here, we review the ► seizure epidemiology, diagnostic considerations, and treatment options for PTE and develop ► neuromodulation a roadmap for providers encountering this challenging clinical entity. “…the brain may be injured by contusion, laceration, the wake of TBI. -
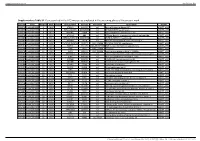
Supplementary Table S1. Genes Printed in the HC5 Microarray Employed in the Screening Phase of the Present Work
Supplementary material Ann Rheum Dis Supplementary Table S1. Genes printed in the HC5 microarray employed in the screening phase of the present work. CloneID Plate Position Well Length GeneSymbol GeneID Accession Description Vector 692672 HsxXG013989 2 B01 STK32A 202374 null serine/threonine kinase 32A pANT7_cGST 692675 HsxXG013989 3 C01 RPS10-NUDT3 100529239 null RPS10-NUDT3 readthrough pANT7_cGST 692678 HsxXG013989 4 D01 SPATA6L 55064 null spermatogenesis associated 6-like pANT7_cGST 692679 HsxXG013989 5 E01 ATP1A4 480 null ATPase, Na+/K+ transporting, alpha 4 polypeptide pANT7_cGST 692689 HsxXG013989 6 F01 ZNF816-ZNF321P 100529240 null ZNF816-ZNF321P readthrough pANT7_cGST 692691 HsxXG013989 7 G01 NKAIN1 79570 null Na+/K+ transporting ATPase interacting 1 pANT7_cGST 693155 HsxXG013989 8 H01 TNFSF12-TNFSF13 407977 NM_172089 TNFSF12-TNFSF13 readthrough pANT7_cGST 693161 HsxXG013989 9 A02 RAB12 201475 NM_001025300 RAB12, member RAS oncogene family pANT7_cGST 693169 HsxXG013989 10 B02 SYN1 6853 NM_133499 synapsin I pANT7_cGST 693176 HsxXG013989 11 C02 GJD3 125111 NM_152219 gap junction protein, delta 3, 31.9kDa pANT7_cGST 693181 HsxXG013989 12 D02 CHCHD10 400916 null coiled-coil-helix-coiled-coil-helix domain containing 10 pANT7_cGST 693184 HsxXG013989 13 E02 IDNK 414328 null idnK, gluconokinase homolog (E. coli) pANT7_cGST 693187 HsxXG013989 14 F02 LYPD6B 130576 null LY6/PLAUR domain containing 6B pANT7_cGST 693189 HsxXG013989 15 G02 C8orf86 389649 null chromosome 8 open reading frame 86 pANT7_cGST 693194 HsxXG013989 16 H02 CENPQ 55166