From99mtc@Citrateand9smtc-Pertechnetateion Netate in Saline Solution and Subsequently Treated with a Sephadex G25 Column
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Carbon Monoxide Down-Regulates Α4β1 Integrin
Chigaev et al. BMC Immunology 2014, 15:52 http://www.biomedcentral.com/1471-2172/15/52 RESEARCH ARTICLE Open Access Carbon monoxide down-regulates α4β1 integrin-specific ligand binding and cell adhesion: a possible mechanism for cell mobilization Alexandre Chigaev1,2,3*, Yelena Smagley1,2,3 and Larry A Sklar1,2,3 Abstract Background: Carbon monoxide (CO), a byproduct of heme degradation, is attracting growing attention from the scientific community. At physiological concentrations, CO plays a role as a signal messenger that regulates a number of physiological processes. CO releasing molecules are under evaluation in preclinical models for the management of inflammation, sepsis, ischemia/reperfusion injury, and organ transplantation. Because of our discovery that nitric oxide signaling actively down-regulates integrin affinity and cell adhesion, and the similarity between nitric oxide and CO-dependent signaling, we studied the effects of CO on integrin signaling and cell adhesion. Results: We used a cell permeable CO releasing molecule (CORM-2) to elevate intracellular CO, and a fluorescent Very Late Antigen-4 (VLA-4, α4β1-integrin)-specific ligand to evaluate the integrin state in real-time on live cells. We show that the binding of the ligand can be rapidly down-modulated in resting cells and after inside-out activation through several Gαi-coupled receptors. Moreover, cell treatment with hemin, a natural source of CO, resulted in comparable VLA-4 ligand dissociation. Inhibition of VLA-4 ligand binding by CO had a dramatic effect on cell-cell interaction in a VLA-4/VCAM-1-dependent cell adhesion system. Conclusions: We conclude that the CO signaling pathway can rapidly down-modulate binding of the VLA-4 -specific ligand. -

Emerging Concepts on the Anti-Inflammatory Actions of Carbon Monoxide-Releasing Molecules (CO-Rms)
Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Roberto Motterlini, Benjamin Haas, Roberta Foresti To cite this version: Roberto Motterlini, Benjamin Haas, Roberta Foresti. Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs).. Medical Gas Research, BioMed Central, 2012, 2 (1), pp.28. 10.1186/2045-9912-2-28. inserm-00769904 HAL Id: inserm-00769904 https://www.hal.inserm.fr/inserm-00769904 Submitted on 3 Jan 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Motterlini et al. Medical Gas Research 2012, 2:28 http://www.medicalgasresearch.com/content/2/1/28 MEDICAL GAS RESEARCH REVIEW Open Access Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs) Roberto Motterlini*, Benjamin Haas and Roberta Foresti* Abstract Carbon monoxide-releasing molecules (CO-RMs) are a class of organometallo compounds capable of delivering controlled quantities of CO gas to cells and tissues thus exerting a broad spectrum of pharmacological effects. CO-RMs containing transition metal carbonyls were initially implemented to mimic the function of heme oxygenase-1 (HMOX1), a stress inducible defensive protein that degrades heme to CO and biliverdin leading to anti-oxidant and anti-inflammatory actions. -

Binding of Immunoglobulin G to Protoporphyrin IX and Its Derivatives: Evidence the Fab Domain Recognizes the Protoporphyrin Ring
antibodies Article Binding of Immunoglobulin G to Protoporphyrin IX and Its Derivatives: Evidence the Fab Domain Recognizes the Protoporphyrin Ring Koichi Orino Laboratory of Veterinary Biochemistry, School of Veterinary Medicine, Kitasato University, Aomori 034-8628, Japan; [email protected]; Tel.: +81-176-23-3471; Fax: +81-176-23-8703 Received: 15 November 2018; Accepted: 27 December 2018; Published: 4 January 2019 Abstract: Immunoglobulin G (IgG) is known to bind zinc via the Fc domain. In this study, biotinylated protoporphyrin IX (PPIX) was incubated with human IgG and then zinc-immobilized Sepharose beads (Zn-beads) were added to the mixture. After washing the beads, the binding of biotinylated PPIX with IgG trapped on Zn-beads was detected using alkaline phosphatase (ALP)-labeled avidin. Human IgG and its Fab domain coated on microtiter plate wells recognized biotin-labeled PPIX and its derivatives, Fe-PPIX and Zn-PPIX, whereas the Fc domain showed some extent of reaction only with Zn-PPIX. When rabbit anti-bovine transferrin (Tf) antibodies were incubated with biotinylated PPIX, the binding of anti-Tf antibodies with apo-Tf was indirectly detected using ALP-labeled avidin, suggesting that even if the antibody is modified with PPIX, the antibody-antigen reaction occurs. These results suggest that the IgG Fab domain recognizes PPIX and its derivatives, probably via the recognition of the PPIX ring. It is unlikely that binding between the Fab domain and PPIX affects the Fc domain-zinc interaction or antigen-antibody reaction. Keywords: antibody; Fab domain; Fc domain; protoporphyrin; zinc 1. Introduction In 1890, von Behring and Kitasato reported the presence of an agent in the blood that functioned as an anti-toxin, neutralizing diphtheria toxin, and this agent was eventually identified as the antibodies that control infection in body tissues [1,2]. -

Quantum of Effectiveness Evidence in FDA's Approval of Orphan Drugs
Quantum of Effectiveness Evidence in FDA’s Approval of Orphan Drugs Cataloguing FDA’s Flexibility in Regulating Therapies for Persons with Rare Disorders by Frank J. Sasinowski, M.S., M.P.H., J.D.1 Chairman of the Board National Organization for Rare Disorders One of the key underlying issues facing the development of effective for their intended uses. all drugs, and particularly orphan drugs, is what kind of evi- dence the Food and Drug Administration (FDA) requires for FDA has for many decades acknowledged that there is a need approval. The Federal Food, Drug, and Cosmetic [FD&C] Act for flexibility in applying its standard for approval. For ex- provides that for FDA to grant approval for a new drug, there ample, one of FDA’s regulations states that: “FDA will ap- must be “substantial evidence” of effectiveness derived from prove an application after it determines that the drug meets the “adequate and well-controlled investigations.” This language, statutory standards for safety and effectiveness… While the which dates from 1962, provides leeway for FDA medical re- statutory standards apply to all drugs, the many kinds of drugs viewers to make judgments as to what constitutes “substantial that are subject to the statutory standards and the wide range evidence” of a drug’s effectiveness, that is, of its benefit to of uses for those drugs demand flexibility in applying the stan- patients. dards. Thus FDA is required to exercise its scientific judgment to determine the kind and quantity of data and information an The sole law that applies specifically to orphan drugs, the Or- applicant is required to provide for a particular drug to meet phan Drug Act of 1983, provided financial incentives for drug the statutory standards.” 21 C.F.R. -

Photosensitivity of a Protoporphyrin
Proc. NatI. Acad. Sci. USA Vol. 88, pp. 10520-10524, December 1991 Genetics Photosensitivity of a protoporphyrin-accumulating, light-sensitive mutant (visA) of Escherichia coli K-12 (heme/active oxygen/protoporphyrin IX/ferrochelatase) KENJI NAKAHIGASHI, KoICHI NISHIMURA, KAZUMASA MIYAMOTO, AND HACHIRO INOKUCHI* Department of Biophysics, Faculty of Science, Kyoto University, Sakyo-ku, Kyoto 606, Japan Communicated by Julius Adler, August 19, 1991 ABSTRACT Mutations in the visA gene ofEscherichia coli were isolated from VS200 by mutagenesis with N-methyl- cause the mutant bacteria to die upon illumination with visible N'-nitro-N-nitrosoguanidine (NTG). A hemin-permeable mu- light. We confirmed genetically that the visA gene is a struc- tant was also isolated with NTG. The AsodA::lacZ CmR tural gene for ferrochelatase (protoheme ferro-lyase, EC marker was transduced from QC772 by P1 transduction, as 4.99.1.1). Since other mutations in the genes involved in the described by Miller (6). biosynthesis of heme can cure the photosensitivity, the light- Media and Growth. The basic media used were LB and M9 induced cell death appears to be brought about by the accu- minimal medium (7). Fumarate minimal medium contained mulation of protoporphyrin IX, one of the substrates of fer- mineral salts (8), 1% glycerol, and 50 mM sodium fumarate. rochelatase. When cells are illuminated with visible light, NO3 minimal medium contained mineral salts, 0.5% glycerol, protoporphyrin IX seems to produce an active species ofoxygen 1% potassium nitrate, and 1 ,uM ammonium molybdate. (probably 102) that is harmful to the cells. This defect is the When required, media were supplemented with 0.2% glu- same as that associated with the human disease protoporphy- cose, 0.6% sodium succinate, 20 tkg ofa particular amino acid ria. -

Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders
antioxidants Review Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders Stefan W. Ryter Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medical College, 525 East 68th Street, Room M-522, Box 130, New York, NY 10065, USA; [email protected] Received: 19 September 2020; Accepted: 2 November 2020; Published: 19 November 2020 Abstract: Heme oxygenase-1 (HO-1) is an inducible stress protein that catalyzes the oxidative conversion of heme to carbon monoxide (CO), iron, and biliverdin (BV), the latter of which is converted to bilirubin (BR) by biliverdin reductase. HO-1 has been implicated as a cytoprotectant in various models of acute organ injury and disease (i.e., lung, kidney, heart, liver). Thus, HO-1 may serve as a general therapeutic target in inflammatory diseases. HO-1 may function as a pleiotropic modulator of inflammatory signaling, via the removal of heme, and generation of its enzymatic degradation-products. Iron release from HO activity may exert pro-inflammatory effects unless sequestered, whereas BV/BR have well-established antioxidant properties. CO, derived from HO activity, has been identified as an endogenous mediator that can influence mitochondrial function and/or cellular signal transduction programs which culminate in the regulation of apoptosis, cellular proliferation, and inflammation. Much research has focused on the application of low concentration CO, whether administered in gaseous form by inhalation, or via the use of CO-releasing molecules (CORMs), for therapeutic benefit in disease. The development of novel CORMs for their translational potential remains an active area of investigation. -

Hemin and Chlorophyll— the Two Most Important Pigments for Life on Earth1
THE OHIO JOURNAL OF SCIENCE VOL. LVI JULY, 1956 No. 4 HEMIN AND CHLOROPHYLL— THE TWO MOST IMPORTANT PIGMENTS FOR LIFE ON EARTH1 PAUL ROTHEMUND The Ohio State University, Columbus, 10, and Muskingum College, New Concord, Ohio Two chemical processes are the prerequisites for all life on earth: the absorption of some of the energy from the sun in the green plants and its transformation into carbon compounds on one hand, and the use of the chemical energy of these compounds by animals in controlled decomposition reactions on the other. From the chemist's point of view the green leaf is a veritable chemical labora- tory: carbon dioxide from the air, and water and inorganic salts from the soil are the raw material, the visible portion of the sun radiation furnishes the energy, and the numerous complex constituents of the plant represent the manufactured products. Some of the substances synthesized are structural matter, like cellulose in the wood, or cork in the bark, others are food reserves, as starch in the grains of corn or wheat, or in potatoes. Of the many other materials produced in the green plant only a few may be enumerated here, sugars, fats, oils and waxes, proteins and nucleic acids, fibers like cotton or hemp, vitamins, hormones, indigo and other dyes, latex for producing rubber, alkaloids like the nicotin in tobacco leaves, valuable medicinally used compounds, such as quinine, cocaine, and morphine, and—most important—the green pigment chlorophyll. "Photo- synthesis", or the "assimilation of carbon dioxide" is the biochemical process, in which simply constructed and relatively inert inorganic compounds are built up into the highly complex, reactive and sensitive organic compounds, which characterize living matter. -
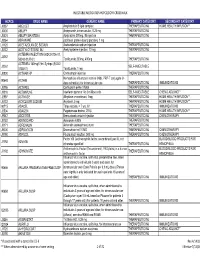
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Perioperative Medication Management - Adult/Pediatric - Inpatient/Ambulatory Clinical Practice Guideline
Effective 6/11/2020. Contact [email protected] for previous versions. Perioperative Medication Management - Adult/Pediatric - Inpatient/Ambulatory Clinical Practice Guideline Note: Active Table of Contents – Click to follow link INTRODUCTION........................................................................................................................... 3 SCOPE....................................................................................................................................... 3 DEFINITIONS .............................................................................................................................. 3 RECOMMENDATIONS ................................................................................................................... 4 METHODOLOGY .........................................................................................................................28 COLLATERAL TOOLS & RESOURCES..................................................................................................31 APPENDIX A: PERIOPERATIVE MEDICATION MANAGEMENT .................................................................32 APPENDIX B: TREATMENT ALGORITHM FOR THE TIMING OF ELECTIVE NONCARDIAC SURGERY IN PATIENTS WITH CORONARY STENTS .....................................................................................................................58 APPENDIX C: METHYLENE BLUE AND SEROTONIN SYNDROME ...............................................................59 APPENDIX D: AMINOLEVULINIC ACID AND PHOTOTOXICITY -

Injections: Drugs EH Policy
inject drug e-h 1 Injections: Drugs E-H Policy Page updated: September 2020 This section outlines policy related to billing for injection services, listed in alphabetical order by generic drug name or drug type. For general billing policy information regarding injections services, refer to the Injections: An Overview section in this manual. Additional policy information for injection services can be found in the following sections of this manual: • Injections: Drugs A–D Policy • Injections: Drugs I–M Policy • Injections: Drugs N–R Policy • Injections: Drugs S–Z Policy • Injections: Hydration • Immunizations Part 2 – Injections: Drugs E-H Policy inject drug e-h 2 Page updated: September 2020 Ecallantide Hereditary angioedema (HAE) is a rare genetic disorder caused by mutations to C1-esterase-inhibitor (C1-INH) located on chromosome 11q and inherited as an autosomal dominant trait. HAE is characterized by low levels of C1-INH activity and low levels of C4. C1-INH functions to regulate the activation of the complement and intrinsic coagulation pathways and is a major endogenous inhibitor of plasma kallikrein. The kallikrein-kinin system is a complex proteolytic cascade involved in the initiation of both inflammatory and coagulation pathways. One critical aspect of this pathway is the conversion of High Molecular Weight (HMW) kininogen to bradykinin by the protease plasma kallikrein. In HAE, normal regulation of plasma kallikrein activity and the classical complement cascade is therefore not present. During attacks, unregulated activity of plasma kallikrein results in excessive bradykinin generation. Bradykinin is a vasodilator which is thought by some to be responsible for the characteristic HAE symptoms of localized swelling, inflammation and pain. -

2019 Table of Drugs
2019 Table of Drugs Questions regarding coding and billing guidance should be submitted to the insurer in whose jurisdiction a claim would be filed. For private sector health insurance systems, please contact the individual private insurance entity. For Medicaid systems, please contact the Medicaid Agency in the state in which the claim is being filed. For Medicare, contact the Medicare contractor. IA - Intra-arterial administration IV - Intravenous administration IM - Intramuscular administration IT - Intrathecal SC - Subcutaneous administration INH - Administration by inhaled solution VAR - Various routes of administration OTH - Other routes of administration ORAL - Administered orally Intravenous administration includes all methods, such as gravity infusion, injections, and timed pushes. The ‘VAR’ posting denotes various routes of administration and is used for drugs that are commonly administered into joints, cavities, tissues, or topical applications, in addition to other parenteral administrations. Listings posted with ‘OTH’ indicate other administration methods, such as suppositories or catheter injections. A Abatacept 10 mg IV J0129 Abbokinase, see Urokinase Abbokinase, Open Cath, see Urokinase Abciximab 10 mg IV J0130 Abelcet, see Amphotericin B Lipid Complex ABLC, see Amphotericin B AbobotulinumtoxintypeA 5 units IM J0586 Acetaminophen 10 mg IV J0131 Acetazolamide sodium up to 500 mg IM, IV J1120 Acetylcysteine, IVection 100 mg IV J0132 Acetylcysteine, unit dose form per gram INH J7604, J7608 1 Achromycin, see Tetracycline Actemra, -

Heme: a Versatile Signaling Molecule Controlling the Activities of Diverse Regulators Ranging from Transcription Factors to MAP Kinases
Sarah M Mense and Li Zhang npg Cell Research (2006): 681-692 npg681 © 2006 IBCB, SIBS, CAS All rights reserved 1001-0602/06 $ 30.00 REVIEW www.nature.com/cr Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases Sarah M Mense1, Li Zhang1 1Department of Environmental Health Sciences, Columbia University, Mailman School of Public Health, 60 Haven Avenue, B-106, New York, NY 10032, USA Heme (iron protoporphyrin IX) is an essential molecule for numerous living organisms. Not only does it serve as a prosthetic group in enzymes, it also acts as a signaling molecule that controls diverse molecular and cellular processes ranging from signal transduction to protein complex assembly. Deficient heme synthesis or function impacts the hema- topoietic, hepatic and nervous systems in humans. Recent studies have revealed a series of heme-regulated transcription factors and signal transducers including Hap1, a heme-activated transcription factor that mediates the effects of oxygen on gene transcription in the yeast Saccharomyces cerevisiae; Bach1, a transcriptional repressor that is negatively regu- lated by heme in mammalian cells; IRR, an iron regulatory protein that mediates the iron-dependant regulation of heme synthesis in the bacterium Bradyrhizobium japonicum; and heme-regulated inhibitor, an eucaryotic initiation factor 2a kinase that coordinates protein synthesis with heme availability in reticulocytes. In this review, we summarize the current knowledge about how heme controls the activity of these transcriptional regulators and signal transducers, and discuss diseases associated with defective heme synthesis, degradation and function. Cell Research (2006) 16:681-692.