Liquid Nitrogen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 5 Measures of Humidity Phases of Water
Chapter 5 Atmospheric Moisture Measures of Humidity 1. Absolute humidity 2. Specific humidity 3. Actual vapor pressure 4. Saturation vapor pressure 5. Relative humidity 6. Dew point Phases of Water Water Vapor n o su ti b ra li o n m p io d a a at ep t v s o io e en s n d it n io o n c freezing Liquid Water Ice melting 1 Coexistence of Water & Vapor • Even below the boiling point, some water molecules leave the liquid (evaporation). • Similarly, some water molecules from the air enter the liquid (condense). • The behavior happens over ice too (sublimation and condensation). Saturation • If we cap the air over the water, then more and more water molecules will enter the air until saturation is reached. • At saturation there is a balance between the number of water molecules leaving the liquid and entering it. • Saturation can occur over ice too. Hydrologic Cycle 2 Air Parcel • Enclose a volume of air in an imaginary thin elastic container, which we will call an air parcel. • It contains oxygen, nitrogen, water vapor, and other molecules in the air. 1. Absolute Humidity Mass of water vapor Absolute humidity = Volume of air The absolute humidity changes with the volume of the parcel, which can change with temperature or pressure. 2. Specific Humidity Mass of water vapor Specific humidity = Total mass of air The specific humidity does not change with parcel volume. 3 Specific Humidity vs. Latitude • The highest specific humidities are observed in the tropics and the lowest values in the polar regions. -

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

Physics, Chapter 17: the Phases of Matter
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 17: The Phases of Matter Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 17: The Phases of Matter" (1958). Robert Katz Publications. 165. https://digitalcommons.unl.edu/physicskatz/165 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 17 The Phases of Matter 17-1 Phases of a Substance A substance which has a definite chemical composition can exist in one or more phases, such as the vapor phase, the liquid phase, or the solid phase. When two or more such phases are in equilibrium at any given temperature and pressure, there are always surfaces of separation between the two phases. In the solid phase a pure substance generally exhibits a well-defined crystal structure in which the atoms or molecules of the substance are arranged in a repetitive lattice. Many substances are known to exist in several different solid phases at different conditions of temperature and pressure. These solid phases differ in their crystal structure. Thus ice is known to have six different solid phases, while sulphur has four different solid phases. -

Compressed Liquid Gases (Cryogens) Laboratory Safe Work Practices
Compressed Liquid Gases (Cryogens) Laboratory Safe Work Practices Source: Section 3.9 of Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories: Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel; excerpted by Vanderbilt OCRS, 10.2020. Compressed Liquid Gases (Cryogens) Cryogenic liquids are liquefied gases that have a normal boiling point below -238°F (-150°C). Liquid nitrogen is used in the microbiology laboratory to freeze and preserve cells and virus stocks. The electron microscopy laboratory, frozen section suites, and grossing stations for surgical pathology frequently use liquid nitrogen; some laboratories also use liquid helium. The principal hazards associated with handling cryogenic fluids include cold contact burns and freezing, asphyxiation, explosion and material embrittlement. Cold contact burns and freezing • Liquid nitrogen is dangerously cold (-320°F [-196°C]), and • Wear loose-fitting thermal gloves with elbow-length cuffs skin contact with either the liquid or gas phase can when filling dewars. Ensure that gloves are loose enough to immediately cause frostbite. At -450°F (-268°C), liquid be thrown off quickly if they contact the liquid. helium is dangerous and cold enough to solidify atmospheric • Never place gloved hands into liquid nitrogen or into the air. liquid nitrogen stream when filling dewars. Gloves are not • Always wear eye protection (face shield over safety goggles). rated for this type of exposure. Insulated gloves are The eyes are extremely sensitive to freezing, and liquid designed to provide short-term protection during handling of nitrogen or liquid nitrogen vapors can cause eye damage. hoses or dispensers and during incidental contact with the • Do not allow any unprotected skin to contact uninsulated liquid. -

Cryogenics – the Basics Or Pre-Basics Lesson 1 D
Cryogenics – The Basics or Pre-Basics Lesson 1 D. Kashy Version 3 Lesson 1 - Objectives • Look at common liquids and gases to get a feeling for their properties • Look at Nitrogen and Helium • Discuss Pressure and Temperature Scales • Learn more about different phases of these fluids • Become familiar with some cryogenic fluids properties Liquids – Water (a good reference) H2O density is 1 g/cc 10cm Total weight 1000g or 1kg (2.2lbs) Cube of water – volume 1000cc = 1 liter Liquids – Motor Oil 10cm 15W30 density is 0.9 g/cc Total weight 900g or 0.9 kg (2lbs) Cube of motor oil – volume 1000cc = 1 liter Density can and usually does change with temperature 15W30 Oil Properties Density Curve Density scale Viscosity scale Viscosity Curve Water density vs temperature What happens here? What happens here? Note: This plot is for SATURATED Water – Discussed soon Water and Ice Water Phase Diagram Temperature and Pressure scales • Fahrenheit: 32F water freezes 212 water boils (at atmospheric pressure) • Celsius: 0C water freezes and 100C water boils (again at atmospheric pressure) • Kelvin: 273.15 water freezes and 373.15 water boils (0K is absolute zero – All motion would stop even electrons around a nucleus) • psi (pounds per square in) one can reference absolute pressure or “gage” pressure (psia or psig) • 14.7psia is one Atmosphere • 0 Atmosphere is absolute vacuum, and 0psia and -14.7psig • Standard Temperature and Pressure (STP) is 20C (68F) and 1 atm Temperature Scales Gases– Air Air density is 1.2kg/m3 => NO Kidding! 100cm =1m Total weight -

Cryogenic Liquid Nitrogen Vehicles (ZEV's)
International Journal of Scientific and Research Publications, Volume 6, Issue 9, September 2016 562 ISSN 2250-3153 Cryogenic Liquid Nitrogen Vehicles (ZEV’S) K J Yogesh Department Of Mechanical Engineering, Jain Engineering College, Belagavi Abstract- As a result of widely increasing air pollution available zero emission vehicle (ZEV) meeting it's standards are throughout the world & vehicle emissions having a major the electrically recharged ones, however these vehicles are also contribution towards the same, it makes its very essential to not a great success in the society due to its own limitations like engineer or design an alternative to the present traditional initial cost, slow recharge, speeds etc. Lead acid & Ni-Cd gasoline vehicles. Liquid nitrogen fueled vehicles can act as an batteries are the past of major technologies in the electric excellent alternative for the same. Liquefied N2 at cryogenic vehicles. They exhibit specific energy in the range of 30-40 W- temperatures can replace conventional fuels in cryogenic heat hr/kg. Lead- acid batteries take hours to recharge & the major engines used as a propellant. The ambient temperature of the drawback of the batteries in all the cases is their replacement surrounding vaporizes the liquid form of N2 under pressure & periodically. This directly/indirectly increases the operating cost leads to the formation of compressed N2 gas. This gas actuates a when studied carefully & thereby not 100% acceptable. pneumatic motor. A combination of multiple reheat open Recent studies make it clear that the vehicles using liquid Rankine cycle & closed Brayton cycle are involved in the nitrogen as their means provide an excellent alternative before process to make use of liquid N2 as a non-polluting fuel. -

Capacity Enhancement of Indigenous Expansion Engine Based Helium Liquefier
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Capacity enhancement of indigenous expansion engine based helium liquefier To cite this article: R S Doohan et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 171 012011 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.8 on 27/09/2021 at 00:16 ICECICMC IOP Publishing IOP Conf. Series: Materials Science and Engineering 171 (2017) 012011 doi:10.1088/1757-899X/171/1/012011 International Conference on Recent Trends in Physics 2016 (ICRTP2016) IOP Publishing Journal of Physics: Conference Series 755 (2016) 011001 doi:10.1088/1742-6596/755/1/011001 Capacity enhancement of indigenous expansion engine based helium liquefier R S Doohan1, P K Kush1, G Maheshwari2 1Raja Ramanna Centre for Advanced Technology, Indore, Madhya Pradesh India. 2Institute of Engineering and Technology, DAVV, Indore, Madhya Pradesh, India. E-mail: [email protected] Abstract. Development of technology and understanding for large capacity helium refrigeration and liquefaction at helium temperature is indispensable for coming-up projects. A new version of helium liquefier designed and built to provide approximately 35 liters of liquid helium per hour. The refrigeration capacity of this reciprocating type expansion engine machine has been increased from its predecessor version with continuous improvement and deficiency debugging. The helium liquefier has been built using components by local industries including cryogenic Aluminum plate fin heat exchangers. Two compressors with nearly identical capacity have been deployed for the operation of system. Together they consume about 110 kW of electric power. The system employs liquid Nitrogen precooling to enhance liquid Helium yield. -

Pressure Vs Temperature (Boiling Point)
Boiling Points and Vapor Pressure Background Boiling Points and Vapor Pressure Background: Definitions Vapor Pressure: The equilibrium pressure of a vapor above its liquid; the pressure of the vapor resulting from the evaporation of a liquid above a sample of the liquid in a closed container. Boiling Point: The temperature at which the vapor pressure of a liquid is equal to the atmospheric (or applied) pressure. As the temperature of the liquid increases, the vapor pressure will increase, as seen below: https://www.chem.purdue.edu/gchelp/liquids/vpress.html Vapor pressure is interpreted in terms of molecules of liquid converting to the gaseous phase and escaping into the empty space above the liquid. In order for the molecules to escape, the intermolecular forces (Van der Waals, dipole- dipole, and hydrogen bonding) have to be overcome, which requires energy. This energy can come in the form of heat, aka increase in temperature. Due to this relationship between vapor pressure and temperature, the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure. The graph below illustrates this relationship using common solvents and some terpenes: Pressure vs Temperature (boiling point) Ethanol Heptane Isopropyl Alcohol B-myrcene 290.0 B-caryophyllene d-Limonene Linalool Pulegone 270.0 250.0 1,8-cineole (eucalyptol) a-pinene a-terpineol terpineol-4-ol 230.0 p-cymene 210.0 190.0 170.0 150.0 130.0 110.0 90.0 Temperature (˚C) Temperature 70.0 50.0 30.0 10 20 30 40 50 60 70 80 90 100 200 300 400 500 600 760 10.0 -10.0 -30.0 Pressure (torr) 1 Boiling Points and Vapor Pressure Background As a very general rule of thumb, the boiling point of many liquids will drop about 0.5˚C for a 10mmHg decrease in pressure when operating in the region of 760 mmHg (atmospheric pressure). -
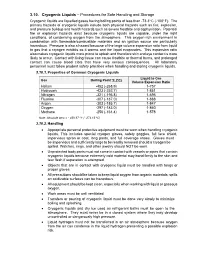
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic Liquids Are Liquefied Gases Having Boiling Points of Less Than -73.3O C (-100O F)
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic liquids are liquefied gases having boiling points of less than -73.3o C (-100o F). The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure buildup and health hazards such as severe frostbite and asphyxiation. Potential fire or explosion hazards exist because cryogenic liquids are capable, under the right conditions, of condensing oxygen from the atmosphere. This oxygen-rich environment in combination with flammable/combustible materials and an ignition source are particularly hazardous. Pressure is also a hazard because of the large volume expansion ratio from liquid to gas that a cryogen exhibits as it warms and the liquid evaporates. This expansion ratio also makes cryogenic liquids more prone to splash and therefore skin and eye contact is more likely to occur. Contact with living tissue can cause frostbite or thermal burns, and prolonged contact can cause blood clots that have very serious consequences. All laboratory personnel must follow prudent safety practices when handling and storing cryogenic liquids. 3.10.1. Properties of Common Cryogenic Liquids Liquid to Gas Gas Boiling Point oF (oC) Volume Expansion Ratio Helium -452 (-268.9) 1-757 Hydrogen -423 (-252.7) 1-851 Nitrogen -321 (-195.8) 1-696 Fluorine -307 (-187.0) 1-888 Argon -303 (-185.7) 1-847 Oxygen -297 (-183.0) 1-860 Methane -256 (-161.4) 1-578 Note: Absolute zero = - 459.67 oF (- 273.15 oC) 3.10.2. Handling Appropriate personal protective equipment must be worn when handling cryogenic liquids. -

ESSENTIALS of METEOROLOGY (7Th Ed.) GLOSSARY
ESSENTIALS OF METEOROLOGY (7th ed.) GLOSSARY Chapter 1 Aerosols Tiny suspended solid particles (dust, smoke, etc.) or liquid droplets that enter the atmosphere from either natural or human (anthropogenic) sources, such as the burning of fossil fuels. Sulfur-containing fossil fuels, such as coal, produce sulfate aerosols. Air density The ratio of the mass of a substance to the volume occupied by it. Air density is usually expressed as g/cm3 or kg/m3. Also See Density. Air pressure The pressure exerted by the mass of air above a given point, usually expressed in millibars (mb), inches of (atmospheric mercury (Hg) or in hectopascals (hPa). pressure) Atmosphere The envelope of gases that surround a planet and are held to it by the planet's gravitational attraction. The earth's atmosphere is mainly nitrogen and oxygen. Carbon dioxide (CO2) A colorless, odorless gas whose concentration is about 0.039 percent (390 ppm) in a volume of air near sea level. It is a selective absorber of infrared radiation and, consequently, it is important in the earth's atmospheric greenhouse effect. Solid CO2 is called dry ice. Climate The accumulation of daily and seasonal weather events over a long period of time. Front The transition zone between two distinct air masses. Hurricane A tropical cyclone having winds in excess of 64 knots (74 mi/hr). Ionosphere An electrified region of the upper atmosphere where fairly large concentrations of ions and free electrons exist. Lapse rate The rate at which an atmospheric variable (usually temperature) decreases with height. (See Environmental lapse rate.) Mesosphere The atmospheric layer between the stratosphere and the thermosphere. -

Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please Show All Work for Credit
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1. 1 2. –1 A least squares fit of ln P versus 1/T gives the result Hvaporization = 25.28 kJ mol . 3. Assuming constant pressure and temperature, and that the surface area of the protein is reduced by 25% due to the hydrophobic interaction: 2 G 0.25 N A 4 r Convert to per mole, ↓determine size per molecule 4 r 3 V M N 0.73mL/ g 60000g / mol (6.02 1023) 3 2 2 A r 2.52 109 m 2 23 9 2 G 0.25 N A 4 r 0.0720N / m 0.25 6.02 10 / mol (4 ) (2.52 10 m) 865kJ / mol We think this is a reasonable approach, but the value seems high 2 4. The vapor pressure of an unknown solid is approximately given by ln(P/Torr) = 22.413 – 2035(K/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr) = 18.352 – 1736(K/T). a. Calculate Hvaporization and Hsublimation. b. Calculate Hfusion. c. Calculate the triple point temperature and pressure. a) Calculate Hvaporization and Hsublimation. From Equation (8.16) dPln H sublimation dT RT 2 dln P d ln P dT d ln P H T 2 sublimation 11 dT dT R dd TT For this specific case H sublimation 2035 H 16.92 103 J mol –1 R sublimation Following the same proedure as above, H vaporization 1736 H 14.43 103 J mol –1 R vaporization b. -

Chapter Two Properties of Ammonia
Chapter Two Properties of Ammonia Physical Properties General Anhydrous ammonia exists as either a colorless gas, colorless liquid, or white solid, depending on its pressure and temperature. In nearly all commonly encountered situations, it exists as either a liquid or a gas. The gas is less dense than air and the liquid is less dense than water at standard conditions. Ammonia gas (vapor) diffuses readily in air and the liquid is highly soluble in water with an accompanying release of heat. Ammonia exhibits classical saturation relationships whereby pressure and temperature are directly related so long as both the vapor and liquid phase are present. It does have a critical pressure and temperature. At atmospheric pressure, a closed container of ammonia vapor and liquid will be in equilibrium at a temperature of –28°F [–33°C]. It should be noted however that if liquid ammonia is spilled or released to the atmosphere at normal temperatures, the resultant pool of boiling liquid will be significantly colder than –28°F due to the law of partial pressures (the partial pressure of the ammonia vapor in the air near the liquid surface will be less than atmospheric pressure). The following table provides some of the important physical properties of ammonia. TABLE 2-1 Physical Properties of Ammonia Property Condition Value (IP) Value (SI) Molecular Weight 17.03 17.03 Color None None Physical State Room Temp Gas Gas Freezing Point P=1 atm –108°F –78°C Boiling Point P=1 atm –28.1°F –33.3°C Critical Pressure 1657 psia 11,410 kPa Critical Temp 271°F