Chapter 6-1 Onychophora
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caesarean Section Or Vaginal Delivery in the 21St Century
CAESAREAN SECTION OR VAGINAL DELIVERY IN THE 21ST CENTURY ntil the 20th Century, caesarean fluid embolism. The absolute risk of trans-placentally to the foetus, prepar- section (C/S) was a feared op- death with C/S in high and middle- ing the foetus to adopt its mother’s Ueration. The ubiquitous classical resource settings is between 1/2000 and microbiome. C/S interferes with neonatal uterine incision meant high maternal 1/4000 (2, 3). In subsequent pregnancies, exposure to maternal vaginal and skin mortality from bleeding and future the risk of placenta previa, placenta flora, leading to colonization with other uterine rupture. Even with aseptic surgi- accreta and uterine rupture is increased. environmental microbes and an altered cal technique, sepsis was common and These conditions increase maternal microbiome. Routine antibiotic exposure lethal without antibiotics. The operation mortality and severe maternal morbid- with C/S likely alters this further. was used almost solely to save the life of ity cumulatively with each subsequent Microbial exposure and the stress of a mother in whom vaginal delivery was C/S. This is of particular importance to labour also lead to marked activation extremely dangerous, such as one with women having large families. of immune system markers in the cord placenta previa. Foetal death and the use blood of neonates born vaginally or by of intrauterine foetal destructive proce- Maternal Benefits C/S after labour. These changes are absent dures, which carry their own morbidity, C/S has a modest protective effect against in the cord blood of neonates born by were often preferable to C/S. -

De Los Reptiles Del Yasuní
guía dinámica de los reptiles del yasuní omar torres coordinador editorial Lista de especies Número de especies: 113 Amphisbaenia Amphisbaenidae Amphisbaena bassleri, Culebras ciegas Squamata: Serpentes Boidae Boa constrictor, Boas matacaballo Corallus hortulanus, Boas de los jardines Epicrates cenchria, Boas arcoiris Eunectes murinus, Anacondas Colubridae: Dipsadinae Atractus major, Culebras tierreras cafés Atractus collaris, Culebras tierreras de collares Atractus elaps, Falsas corales tierreras Atractus occipitoalbus, Culebras tierreras grises Atractus snethlageae, Culebras tierreras Clelia clelia, Chontas Dipsas catesbyi, Culebras caracoleras de Catesby Dipsas indica, Culebras caracoleras neotropicales Drepanoides anomalus, Culebras hoz Erythrolamprus reginae, Culebras terrestres reales Erythrolamprus typhlus, Culebras terrestres ciegas Erythrolamprus guentheri, Falsas corales de nuca rosa Helicops angulatus, Culebras de agua anguladas Helicops pastazae, Culebras de agua de Pastaza Helicops leopardinus, Culebras de agua leopardo Helicops petersi, Culebras de agua de Peters Hydrops triangularis, Culebras de agua triángulo Hydrops martii, Culebras de agua amazónicas Imantodes lentiferus, Cordoncillos del Amazonas Imantodes cenchoa, Cordoncillos comunes Leptodeira annulata, Serpientes ojos de gato anilladas Oxyrhopus petolarius, Falsas corales amazónicas Oxyrhopus melanogenys, Falsas corales oscuras Oxyrhopus vanidicus, Falsas corales Philodryas argentea, Serpientes liana verdes de banda plateada Philodryas viridissima, Serpientes corredoras -

Introduction Methods Results
Papers and Proceedings of the Royal Society of Tasmania, Volume l 25, 1991 11 ECOLOGY AND CONSERVATION OF TASMANIPATUS BARRETT/ AND T. ANOPHTHALMUS, PARAPATRIC ONYCHOPHORANS (ONYCHOPHORA: PERIPATOPSIDAE) FROM NORTHEASTERN TASMANIA by R. Mesibov and H. Ruhberg (with two text-figures and four plates) MESIBOV, R. & RUHBERC, H., 1991 (20:xii): Ecology and conservation of Tasmanipatus barretti and T anophthalmus, parapacric onychophorans (Onychophora: Peripatopsidae) from northeastern Tasmania. Pap. Proc. R. Soc. Tasm. 125: 11- 16. https://doi.org/10.26749/rstpp.125.11 ISSN 0080- 4703. PO Box 431, Smithton, Tasmania, Australia 7330 (RM); and Zoologischcs lnstitut und Zoologischcs Museum, Universitat Hamburg, Martin-Luther-King-Platz 3, D-2000 Hamburg 13, Germany (HR). Tasmanipatus barretti and T anophthalmus are parapatrically distributed in northeasternTasmania with known ranges of about 600 km2 and 200 km2 respectively. Both species occur in wet sclerophyll forest. Both appear to tolerate habirat disturbance such as occasional bushfires, but are eliminated by forestclearing foragriculture or pine plantations. Both are found in forest reserves, and are to be furtherprotected by a habitat management programme devised by the Tasmanian Forestry Commission. Key Words: Onychophorans, northeastern Tasmania, parapatry, sderophyii forest, conservation. INTRODUCTION and (i) records ofincidental collections by RM during private field trips, 1984-90 (13 localities). Two rare and unusual species of peripatopsid onychophorans At each site visited in studies (a), (b), (d) and (h), have recently been found in northeastern Tasmania. One onychophorans were hunted by gently breaking apart rotting species, Tasmanipatus barretti, locally known as the giant logs and stumps. Less thorough inspections were made velvet worm, is the largest Tasmanian onychophoran. -

Arachnida, Solifugae) with Special Focus on Functional Analyses and Phylogenetic Interpretations
HISTOLOGY AND ULTRASTRUCTURE OF SOLIFUGES Comparative studies of organ systems of solifuges (Arachnida, Solifugae) with special focus on functional analyses and phylogenetic interpretations HISTOLOGIE UND ULTRASTRUKTUR DER SOLIFUGEN Vergleichende Studien an Organsystemen der Solifugen (Arachnida, Solifugae) mit Schwerpunkt auf funktionellen Analysen und phylogenetischen Interpretationen I N A U G U R A L D I S S E R T A T I O N zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) an der Mathematisch-Naturwissenschaftlichen Fakultät der Ernst-Moritz-Arndt-Universität Greifswald vorgelegt von Anja Elisabeth Klann geboren am 28.November 1976 in Bremen Greifswald, den 04.06.2009 Dekan ........................................................................................................Prof. Dr. Klaus Fesser Prof. Dr. Dr. h.c. Gerd Alberti Erster Gutachter .......................................................................................... Zweiter Gutachter ........................................................................................Prof. Dr. Romano Dallai Tag der Promotion ........................................................................................15.09.2009 Content Summary ..........................................................................................1 Zusammenfassung ..........................................................................5 Acknowledgments ..........................................................................9 1. Introduction ............................................................................ -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Ecdysozoan Mitogenomics: Evidence for a Common Origin of the Legged Invertebrates, the Panarthropoda
GBE Ecdysozoan Mitogenomics: Evidence for a Common Origin of the Legged Invertebrates, the Panarthropoda Omar Rota-Stabelli*,1,2, Ehsan Kayal3, Dianne Gleeson4, Jennifer Daub5, Jeffrey L. Boore6, Maximilian J. Telford1, Davide Pisani2, Mark Blaxter5, and Dennis V. Lavrov*,3 1Department of Genetics, Evolution and Environment, University College London, London, United Kingdom 2Department of Biology, National University of Ireland, Maynooth, Maynooth, Co. Kildare, Ireland 3Department of Ecology, Evolution and Organismal Biology, Iowa State University 4EcoGene, Landcare Research New Zealand Ltd., St Johns, Auckland, New Zealand Downloaded from 5Institute of Evolutionary Biology, The University of Edinburgh, Ashworth Laboratories, Edinburgh, United Kingdom 6Genome Project Solutions, Hercules, California *Corresponding author: E-mail: [email protected], [email protected]; [email protected]. Accepted: 26 May 2010 gbe.oxfordjournals.org Abstract Ecdysozoa is the recently recognized clade of molting animals that comprises the vast majority of extant animal species and the most important invertebrate model organisms—the fruit fly and the nematode worm. Evolutionary relationships within the ecdysozoans remain, however, unresolved, impairing the correct interpretation of comparative genomic studies. In particular, the affinities of the three Panarthropoda phyla (Arthropoda, Onychophora, and Tardigrada) and the position of at University of South Carolina on November 30, 2010 Myriapoda within Arthropoda (Mandibulata vs. Myriochelata hypothesis) are among the most contentious issues in animal phylogenetics. To elucidate these relationships, we have determined and analyzed complete or nearly complete mitochondrial genome sequences of two Tardigrada, Hypsibius dujardini and Thulinia sp. (the first genomes to date for this phylum); one Priapulida, Halicryptus spinulosus; and two Onychophora, Peripatoides sp. and Epiperipatus biolleyi; and a partial mitochondrial genome sequence of the Onychophora Euperipatoides kanagrensis. -

Onychophora, Peripatidae) Feeding on a Theraphosid Spider (Araneae, Theraphosidae)
2009. The Journal of Arachnology 37:116–117 SHORT COMMUNICATION First record of an onychophoran (Onychophora, Peripatidae) feeding on a theraphosid spider (Araneae, Theraphosidae) Sidclay C. Dias and Nancy F. Lo-Man-Hung: Museu Paraense Emı´lio Goeldi, Laborato´rio de Aracnologia, C.P. 399, 66017-970, Bele´m, Para´, Brazil. E-mail: [email protected] Abstract. A velvet worm (Peripatus sp., Peripatidae) was observed and photographed while feeding on a theraphosid spider, Hapalopus butantan (Pe´rez-Miles, 1998). The present note is the first report of an onychophoran feeding on ‘‘giant’’ spider. Keywords: Prey behavior, velvet worm, spider Onychophorans, or velvet worms, are organisms whose behavior on the floor forests (pers. obs.). Onychophorans are capable of preying remains poorly understood due to their cryptic lifestyle (New 1995) on animals their own size, although the quantity of glue used in an attack and by the fact they are rare in the Neotropics (Mcglynn & Kelley increases up to about 80% of the total capacity for larger prey (Read & 1999). Consequently reports on hitherto unknown aspects of the Hughes 1987). It may be that encounters with larger prey items, such as biology and life history of onychophorans are urgently needed. that observed by us, are more common than previously supposed. Onychophorans are almost all carnivores that prey on small invertebrates such as snails, isopods, earth worms, termites, and other ACKNOWLEDGMENTS small insects (Hamer et al. 1997). They are widely distributed in Thanks to G. Machado (USP), T.A. Gardner (Universidade southern hemisphere temperate regions and in the tropics (Reinhard Federal de Lavras), and C.A. -

Vocabulary: Sharks
Grades 11-12 - Vocabulary: Sharks Dermal Denticles – Tiny tooth-shaped scales that cover a shark’s body. Dermal Denticles have the same structure as teeth - enamel, dentine, pulp, epidermis, and dermis. Counter Shading - Having a dark dorsal or upper side and a lighter colored underside. Lateral Line – A row of sensors used by sharks and other fish, which detect vibrations. Cartilage – The material that makes up a shark’s skeleton (not bone), and is also found in our ears and nose. Basihyal - A sharks tongue, composed of a small piece of cartilage on the bottom of a sharks’ mouth. Carnivore - An animal that eats meat. Megalodon - An ancient shark that lived between 5 and 1.6 million years ago. Serrated Tooth - A tooth with a jagged edge that is used for sawing. Dorsal Fin - Primary fin located on the back of fishes and certain marine mammals. Pectoral Fins - Either of the anterior pairs of fins. Barbels - Sensory projections near the nostrils and mouth of some sharks, i.e. nurse sharks. They are whisker-like feelers used to taste and feel. Gills - Respiratory organs that fish use to absorb oxygen from the water in order to breathe. Snout - The tip of a shark’s head. Pup - A newly born or hatched shark. Claspers - Two finger like projections on the rear underside of male sharks. Ampullae of Lorenzini - Pores scattered about the head of sharks that are filled with a jellylike substance that can sense temperature change and weak electrical impulses given off by sick prey. Fusiform – A streamlined, oval shape body. -

The Early Amber Caught the Wormª a 100 Million-Year-Old Onychophoran Reveals Past Migrations
The early amber caught the wormª A 100 million-year-old onychophoran reveals past migrations The split of the supercontinent Pangaea into southern Gondwana and northern Laurasia divided the fauna of these two regions. Therefore, the present-day occurrence of supposedly Gondwanan organisms in Laurasian-derived regions remains a puzzle of palaeobiogeographical history. We studied the oldest amber-embedded species of velvet worms (Onychophora) in order to illuminate the colonisation of Southeast Asia by Gondwanan lineages of these animals. Our results indicate that an early Eurogondwanan migration is the most likely scenario for Onychophora, while an ‘Out-of-India’ colonisation of Southeast Asia would instead be incompatible with the age of the amber fossil studied. This suggests a recent colonisation of India by onychophorans and refutes their Gondwanan relict status in this region. Burmese amber from Myanmar is known not only for its hypothesis recently named the Eurogondwana model [4]. physical beauty but also for preserving one of the richest Alternatively, since onychophorans are poor dispersers, it palaeobiota in the world, being arguably the most relevant was proposed that the Indian subcontinent acted as a raft fossil resin for studying terrestrial diversity during the mid- during its northward drift and brought Gondwanan species of Cretaceous period, approximately 100 million years ago [1]. Peripatidae to Southeast Asia after the so-called ‘India–Asia Among the most consequential organisms found in Burmese collision’, a biogeographical model commonly called ‘Out– amber is the oldest amber-embedded representative of of–India’ [5]. Accordingly, the only onychophoran species Onychophora — a small group of soft-bodied, terrestrial reported from India, Typhloperipatus williamsoni [6], is invertebrates pivotal for understanding animal evolution and putatively described as being a Gondwanan relict that survived biogeography. -
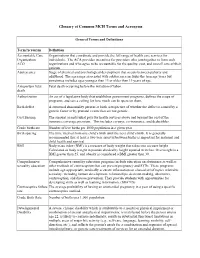
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

A Prospectus for BIOL228 Organismal Biology Basic Information
A prospectus for BIOL228 Organismal Biology Basic information • BIOL228 and 229 succeed 208 (Animal Structure and Function) and 210 (Plant Structure and Function) • Both new units to be offered in S1 2017 • Prereqs are 114 and 115 • 208's enrolment in S1 2016 was > 150 Handbook description "This unit explores the biological diversity of plants and animals. Relationships between structure and function are emphasised. The unit also discusses how organisms have adapted to specific environments. There is a strong emphasis on evolutionary processes and how these have generated biological diversity. A comparative approach is taken, with adaptation discussed in the context of evolutionary trees and the fossil record. The unit is suitable for students interested in organismal biology, science education, and research." Handbook description "This unit explores the biological diversity of plants and animals. Relationships between structure and function are emphasised. The unit also discusses how organisms have adapted to specific environments. There is a strong emphasis on evolutionary processes and how these have generated biological diversity. A comparative approach is taken, with adaptation discussed in the context of evolutionary trees and the fossil record. The unit is suitable for students interested in organismal biology, science education, and research." Program-level learning outcomes 1. Explain the theory of evolution and why it can be regarded as the central unifying concept in biology 2. Compare and contrast form and function of key biological units at sub-cellular to ecosystem scales 3. Describe key features of the Australian biota and the processes that have given rise to these 4. Evaluate historical developments in biology, as well as current and contemporary research directions and challenges Unit-specific learning outcomes 1. -

Onychophorology, the Study of Velvet Worms
Uniciencia Vol. 35(1), pp. 210-230, January-June, 2021 DOI: http://dx.doi.org/10.15359/ru.35-1.13 www.revistas.una.ac.cr/uniciencia E-ISSN: 2215-3470 [email protected] CC: BY-NC-ND Onychophorology, the study of velvet worms, historical trends, landmarks, and researchers from 1826 to 2020 (a literature review) Onicoforología, el estudio de los gusanos de terciopelo, tendencias históricas, hitos e investigadores de 1826 a 2020 (Revisión de la Literatura) Onicoforologia, o estudo dos vermes aveludados, tendências históricas, marcos e pesquisadores de 1826 a 2020 (Revisão da Literatura) Julián Monge-Nájera1 Received: Mar/25/2020 • Accepted: May/18/2020 • Published: Jan/31/2021 Abstract Velvet worms, also known as peripatus or onychophorans, are a phylum of evolutionary importance that has survived all mass extinctions since the Cambrian period. They capture prey with an adhesive net that is formed in a fraction of a second. The first naturalist to formally describe them was Lansdown Guilding (1797-1831), a British priest from the Caribbean island of Saint Vincent. His life is as little known as the history of the field he initiated, Onychophorology. This is the first general history of Onychophorology, which has been divided into half-century periods. The beginning, 1826-1879, was characterized by studies from former students of famous naturalists like Cuvier and von Baer. This generation included Milne-Edwards and Blanchard, and studies were done mostly in France, Britain, and Germany. In the 1880-1929 period, research was concentrated on anatomy, behavior, biogeography, and ecology; and it is in this period when Bouvier published his mammoth monograph.