Ya Tsie BCPP Summary and Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NGAMILAND SUSTAINABLE LAND MANAGEMENT PROJECT “Mainstreaming SLM in Rangeland Areas of Ngamiland- District Landscapes for Improved Livelihoods”
NGAMILAND SUSTAINABLE LAND MANAGEMENT PROJECT “Mainstreaming SLM in Rangeland Areas of Ngamiland- District Landscapes for Improved Livelihoods” Project Steering Committee (PSC) Meeting Thursday 5th July, 2018 Maun Lodge 0900 Hours BOARD DOCUMENTS Page 1 of 42 TABLE OF CONTENTS TABLE OF CONTENTS ..........................................................................................................................2 MEETING AGENDA ..............................................................................................................................3 LIST OF ACRONYMS ............................................................................................................................4 MINUTES OF 2018 QUARTER 1 PSC MEETING .......................................................................................6 ANNEX TO MINUTES ......................................................................................................................... 16 Annex 1: 2018 Quarter 1 Progress Report ....................................................................................... 16 2018 QUARTER 2 PROGRESS REPORT ................................................................................................. 26 Request for Increased ASL ............................................................................................................. 36 Approved Annual Workplan and Budget 2018................................................................................. 37 Page 2 of 42 MEETING AGENDA NGAMILAND SUSTAINABLE LAND MANAGEMENT -

Makgadikgadi Framework Management Plan
Makgadikgadi Framework Management Plan Volume one: Main report November 2010 Republic of Botswana Makgadikgadi Framework Management Plan 2010 Report details This report is volume one of the Makgadikgadi Framework Management Plan prepared for the government by the Department of Environmental Affairs, Ministry of Environment, Wildlife and Tourism in partnership with the Centre for Applied Research. Volume one is the main plan for the Makgadikgadi area. Volume two contains detailed findings of specialist and component studies prepared during 2009/2010. Acknowledgements The MFMP project team is indebted to a large number of institutions, companies, persons and communities that have actively contributed to the projects through: 1. participation in various focus group discussions, project workshops/ meetings and kgotla meetings; 2. being interviewed for the livelihood survey or as a resource person; the provision of data and feedback on draft reports. We sincerely hope that the implementation of the plan will offer a ‘reward’ for these efforts and the time spent on the project. Citation: Department of Environmental Affairs and Centre for Applied Research, 2010. The Makgadikgadi Framework Management Plan. Government of Botswana, Gaborone. Volume one: main report 2 Makgadikgadi Framework Management Plan 2010 Contents List of Figures .......................................................................................................................................... 6 List of Tables .......................................................................................................................................... -

IVIV VV VI Pretoria Pretoria Soweto Soweto 0 125 250 Kmiv IV V V VV V
Earthquake Green Shaking Alert M 6.5, Botswana Origin Time: Mon 2017-04-03 17:40:16 UTC (19:40:16 local) PAGER o o Location: 22.62 S 25.15 E Depth: 11 km Version 1 Created: 40 minutes, 53 seconds after earthquake Estimated Fatalities Green alert for shaking-related fatalities Estimated Economic Losses and economic losses. There is a low likelihood of casualties and damage. 65% 50% 30% 32% 15% 4% 3% 1 100 10,000 1 100 10,000 10 1,000 100,000 10 1,000 100,000 Fatalities USD (Millions) Estimated Population Exposed to Earthquake Shaking ESTIMATED POPULATION - -* 3,776k* 19,253k* 2,733k 54k 2k 0 0 0 EXPOSURE (k = x1000) ESTIMATED MODIFIED MERCALLI INTENSITY PERCEIVED SHAKING Not felt Weak Light Moderate Strong Very Strong Severe Violent Extreme Resistant none none none V. Light Light Moderate Moderate/Heavy Heavy V. Heavy POTENTIAL Structures DAMAGE Vulnerable Structures none none none Light Moderate Moderate/Heavy Heavy V. Heavy V. Heavy *Estimated exposure only includes population within the map area. Population Exposure population per ~1 sq. km from Landscan Structures: Overall, the population in this region resides in structures that are vulnerable to IV 21°E ShakaweShakaweShakawe 24°E HwangeHwange27°E earthquake shaking, though some resistant PandamatengaPandamatengaPandamatenga DeteDete structures exist. LupaneLupaneLupane Historical Earthquakes (with MMI levels): There were no earthquakes with significant NokanengNokaneng InyathiInyathiInyathi ShanganiShanganiShangani population exposure to shaking within a 400 MaunMaun MaunMaun SuaSuaSua -

Land Tenure Reforms and Social Transformation in Botswana: Implications for Urbanization
Land Tenure Reforms and Social Transformation in Botswana: Implications for Urbanization. Item Type text; Electronic Dissertation Authors Ijagbemi, Bayo, 1963- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 06/10/2021 17:13:55 Link to Item http://hdl.handle.net/10150/196133 LAND TENURE REFORMS AND SOCIAL TRANSFORMATION IN BOTSWANA: IMPLICATIONS FOR URBANIZATION by Bayo Ijagbemi ____________________ Copyright © Bayo Ijagbemi 2006 A Dissertation Submitted to the Faculty of the DEPARTMENT OF ANTHROPOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2006 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Bayo Ijagbemi entitled “Land Reforms and Social Transformation in Botswana: Implications for Urbanization” and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 10 November 2006 Dr Thomas Park _______________________________________________________________________ Date: 10 November 2006 Dr Stephen Lansing _______________________________________________________________________ Date: 10 November 2006 Dr David Killick _______________________________________________________________________ Date: 10 November 2006 Dr Mamadou Baro Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Department of Road Transport and Safety Offices
DEPARTMENT OF ROAD TRANSPORT AND SAFETY OFFICES AND SERVICES MOLEPOLOLE • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Transport Inspectorate Tel: 5920148 Fax: 5910620 P/Bag 52 Molepolole Next to Molepolole Police MOCHUDI • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Transport Inspectorate P/Bag 36 Mochudi Tel : 5777127 Fax : 5748542 White House GABORONE Headquarters BBS Mall Plot no 53796 Tshomarelo House (Botswana Savings Bank) 1st, 2nd &3rd Floor Corner Lekgarapa/Letswai Road •Registration & Licensing of vehicles and drivers •Road safety (Public Education) Tel: 3688600/62 Fax : Fax: 3904067 P/Bag 0054 Gaborone GABORONE VTS – MARUAPULA • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Vehicle Examination Tel: 3912674/2259 P/Bag BR 318 B/Hurst Near Roads Training & Roads Maintenance behind Maruapula Flats GABORONE II – FAIRGROUNDS • Registration & Licensing of vehicles and drivers • Driver Examination : Theory Tel: 3190214/3911540/3911994 Fax : P/Bag 0054 Gaborone GABORONE - OLD SUPPLIES • Registration & Licensing of vehicles and drivers • Transport Permits • Transport Inspectorate Tel: 3905050 Fax :3932671 P/Bag 0054 Gaborone Plot 1221, Along Nkrumah Road, Near Botswana Power Corporation CHILDREN TRAFFIC SCHOOL •Road Safety Promotion for children only Tel: 3161851 P/Bag BR 318 B/Hurst RAMOTSWA •Registration & Licensing of vehicles and drivers •Driver Examination (Theory & Practical -

The Hydrology of the Okavango Delta, Botswana—Processes, Data and Modelling
Regional review: the hydrology of the Okavango Delta, Botswana—processes, data and modelling Christian Milzow & Lesego Kgotlhang & Peter Bauer-Gottwein & Philipp Meier & Wolfgang Kinzelbach Abstract The wetlands of the Okavango Delta accom- Introduction modate a multitude of ecosystems with a large diversity in fauna and flora. They not only provide the traditional The Okavango wetlands, commonly called the Okavango livelihood of the local communities but are also the basis Delta, are spread on top of an alluvial fan located in of a tourism industry that generates substantial revenue for northern Botswana, in the western branch of the East the whole of Botswana. For the global community, the African Rift Valley. Waters forming the Okavango River wetlands retain a tremendous pool of biodiversity. As the originate in the highlands of Angola, flow southwards, upstream states Angola and Namibia are developing, cross the Namibian Caprivi-Strip and eventually spread however, changes in the use of the water of the Okavango into the terminal wetlands on Botswanan territory cover- River and in the ecological status of the wetlands are to be ing the alluvial fan (Fig. 1). Whereas the climate in the expected. To predict these impacts, the hydrology of the headwater region is subtropical and humid with an annual Delta has to be understood. This article reviews scientific precipitation of up to 1,300 mm, it is semi-arid in Botswana work done for that purpose, focussing on the hydrological with precipitation amounting to only 450 mm/year in the modelling of surface water and groundwater. Research Delta area. High potential evapotranspiration rates cause providing input data to hydrological models is also over 95% of the wetland inflow and local precipitation to be presented. -

Elephant Social Dynamics, Spatial Ecology and Human Elephant Conflict in the Makgadikgadi Salt Pans and Kalahari Ecosystems
Elephant Social Dynamics, Spatial Ecology and Human Elephant Conflict in the Makgadikgadi Salt Pans and Kalahari Ecosystems August 2009 Submitted to: Department of Wildlife and National Parks, Botswana Funded by: The San Diego Zoo and Elephants Without Borders Michael Chase Elephants Without Borders Po Box 682 Kasane Botswana Tel/Fax: ++267 6250202 Email: [email protected] PROJECT NARRATIVE Background Conservation management plans for wildlife species require accurate and reliable longitudinal information about population size, distribution, demography, reproductive rate and habitat use. However, obtaining detailed data is often hampered due to financial and time constraints imposed on local governments and scientists. Our fundamental aim in this segment of our elephant ecology study in the Kavango Zambezi TFCA is to augment the elephant conservation efforts of the Botswana Government by conducting research on the ecology of elephants in the Makgadikgadi and Kalahari ecosystems to identify factors regulating the spatiotemporal distribution and habitat use of elephants. Our ultimate goal is to share this information with appropriate authorities, communities and the scientific community, in order to mitigate Human Elephant Conflict (HEC) while simultaneously promoting the conservation of African elephants and their natural habitats in Botswana. Our study is unique and timely in that it monitors elephant range patterns in and out of national parks, across international boundaries and in habitats ranging from nearly desert to wetland/riverine environments. No other study has sought to conserve a “flagship” species by incorporating such a large and varied ecosystem. By adopting the most rigorous scientific methods and state-of- the- art techniques to derive estimates of elephant population size and distribution, as well as movement patterns across the region, we will greatly improve our understanding of the dynamic forces regulating elephant life histories, and their interactions with people thereby make significant contributions towards elephant conservation in Botswana. -

Thursday 26 November 2020 the First Meeting of the Second Session of the Twelfth Parliament English Version
THE FIRST MEETING OF THE SECOND SESSION OF THE TWELFTH PARLIAMENT THURSDAY 26 NOVEMBER 2020 ENGLISH VERSION HANSARD NO: 200 THE NATIONAL ASSEMBLY SPEAKER The Hon. Phandu T. C. Skelemani PH, MP. DEPUTY SPEAKER The Hon. Mabuse M. Pule, MP. (Mochudi East) Clerk of the National Assembly - Ms B. N. Dithapo Deputy Clerk of the National Assembly - Mr L. T. Gaolaolwe Learned Parliamentary Counsel - Ms M. Mokgosi Assistant Clerk (E) - Mr R. Josiah CABINET His Excellency Dr M. E. K. Masisi, MP. - President His Honour S. Tsogwane, MP. (Boteti West) - Vice President Minister for Presidential Affairs, Governance and Public Hon. K. N. S. Morwaeng, MP. (Molepolole South) - Administration Hon. K. T. Mmusi, MP. (Gabane-Mmankgodi) - Minister of Defence, Justice and Security Hon. Dr L. Kwape, MP. (Kanye South) - Minister of International Affairs and Cooperation Hon. E. M. Molale, MP. (Goodhope-Mabule ) - Minister of Local Government and Rural Development Hon. K. S. Gare, MP. (Moshupa-Manyana) - Minister of Agricultural Development and Food Security Minister of Environment, Natural Resources Conservation Hon. P. K. Kereng, MP. (Specially Elected) - and Tourism Hon. Dr E. G. Dikoloti MP. (Mmathethe-Molapowabojang) - Minister of Health and Wellness Hon. T.M. Segokgo, MP. (Tlokweng) - Minister of Transport and Communications Hon. K. Mzwinila, MP. (Specially Elected) - Minister of Land Management, Water and Sanitation Services Minister of Youth Empowerment, Sport and Culture Hon. T. M. Rakgare, MP. (Mogoditshane) - Development Hon. A. M. Mokgethi, MP. (Gaborone Bonnington North) - Minister of Nationality, Immigration and Gender Affairs Hon. Dr T. Matsheka, MP. (Lobatse) - Minister of Finance and Economic Development Hon. F. M. M. -

The Magnitude of Termites to the Future of Kimberlite Exploration in Botswana
11th International Kimberlite Conference Extended Abstract No. 11IKC-4555, 2017 The magnitude of termites to the future of kimberlite exploration in Botswana Leon R.M. Daniels, Tshireletso A. Dira and Onesimo Kufandikamwe Pangolin Diamonds (Pty) Limited, Tatitown, Botswana [email protected], [email protected], [email protected] Introduction The majority of the diamond mines in Botswana (Orapa, Jwaneng, Letlhakane, Damsthaa, Lerala and BK11) were discovered as a direct consequence of soil sampling for indicator minerals such as garnet and picroilmenite. Over the past sixty years the application of soil sampling for indicator minerals as a primary exploration tool has diminished while aeromagnetic surveys have increased in popularity. The rate of kimberlite discovery in Botswana over the past fifteen years has declined significantly. The obvious magnetic kimberlites have been discovered. The future of new kimberlite discoveries, mainly poorly magnetic to non-magnetic, is once again dependent on soil sampling for kimberlite indicator minerals. The only mechanism to transport indicators from source to surface through the Kalahari Formation overburden is through termite bioturbation. Soil Sampling History in Botswana Initial soil sampling programmes conducted by De Beers and Central African Selection Trust (CAST) in Botswana (then Bechuanaland) during the mid to late 1950’s were confined to stream sediments in areas where drainage was developed. Concentrates were generated using gold pans. This method was quite ineffective and possibly resulted in Jwaneng being missed in a soil sampling programme conducted in 1962 (Lamont, 2011). A “semi-continuous scoop” sampling method was developed to sample interfluves areas and eventually into the Kalahari sandveld. The gold pan was abandoned as a concentrating tool and a gravitating screen, commonly used by diamond diggers, was introduced to concentrate soil samples between 2mm and 0.5mm in size. -

2011 Population & Housing Census Preliminary Results Brief
2011 Population & Housing Census Preliminary Results Brief For further details contact Census Office, Private Bag 0024 Gaborone: Tel 3188500; Fax 3188610 1. Botswana Population at 2 Million Botswana’s population has reached the 2 million mark. Preliminary results show that there were 2 038 228 persons enumerated in Botswana during the 2011 Population and Housing Census, compared with 1 680 863 enumerated in 2001. Suffice to note that this is the de-facto population – persons enumerated where they were found during enumeration. 2. General Comments on the Results 2.1 Population Growth The annual population growth rate 1 between 2001 and 2011 is 1.9 percent. This gives further evidence to the effect that Botswana’s population continues to increase at diminishing growth rates. Suffice to note that inter-census annual population growth rates for decennial censuses held from 1971 to 2001 were 4.6, 3.5 and 2.4 percent respectively. A close analysis of the results shows that it has taken 28 years for Botswana’s population to increase by one million. At the current rate and furthermore, with the current conditions 2 prevailing, it would take 23 years for the population to increase by another million - to reach 3 million. Marked differences are visible in district population annual growths, with estimated zero 3 growth for Selebi-Phikwe and Lobatse and a rate of over 4 percent per annum for South East District. Most district growth rates hover around 2 percent per annum. High growth rates in Kweneng and South East Districts have been observed, due largely to very high growth rates of villages within the proximity of Gaborone. -
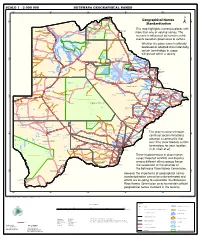
Geographical Names Standardization BOTSWANA GEOGRAPHICAL
SCALE 1 : 2 000 000 BOTSWANA GEOGRAPHICAL NAMES 20°0'0"E 22°0'0"E 24°0'0"E 26°0'0"E 28°0'0"E Kasane e ! ob Ch S Ngoma Bridge S " ! " 0 0 ' ' 0 0 ° Geographical Names ° ! 8 !( 8 1 ! 1 Parakarungu/ Kavimba ti Mbalakalungu ! ± n !( a Kakulwane Pan y K n Ga-Sekao/Kachikaubwe/Kachikabwe Standardization w e a L i/ n d d n o a y ba ! in m Shakawe Ngarange L ! zu ! !(Ghoha/Gcoha Gate we !(! Ng Samochema/Samochima Mpandamatenga/ This map highlights numerous places with Savute/Savuti Chobe National Park !(! Pandamatenga O Gudigwa te ! ! k Savu !( !( a ! v Nxamasere/Ncamasere a n a CHOBE DISTRICT more than one or varying names. The g Zweizwe Pan o an uiq !(! ag ! Sepupa/Sepopa Seronga M ! Savute Marsh Tsodilo !(! Gonutsuga/Gonitsuga scenario is influenced by human-centric Xau dum Nxauxau/Nxaunxau !(! ! Etsha 13 Jao! events based on governance or culture. achira Moan i e a h hw a k K g o n B Cakanaca/Xakanaka Mababe Ta ! u o N r o Moremi Wildlife Reserve Whether the place name is officially X a u ! G Gumare o d o l u OKAVANGO DELTA m m o e ! ti g Sankuyo o bestowed or adopted circumstantially, Qangwa g ! o !(! M Xaxaba/Cacaba B certain terminology in usage Nokaneng ! o r o Nxai National ! e Park n Shorobe a e k n will prevail within a society a Xaxa/Caecae/Xaixai m l e ! C u a n !( a d m a e a a b S c b K h i S " a " e a u T z 0 d ih n D 0 ' u ' m w NGAMILAND DISTRICT y ! Nxai Pan 0 m Tsokotshaa/Tsokatshaa 0 Gcwihabadu C T e Maun ° r ° h e ! 0 0 Ghwihaba/ ! a !( o 2 !( i ata Mmanxotae/Manxotae 2 g Botet N ! Gcwihaba e !( ! Nxharaga/Nxaraga !(! Maitengwe -

Rural Poverty in Botswana. University of Botswana
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Brage INN Gerd Wikan Cash, crops and cattle A study of rural livelihoods in Botswana Høgskolen i Hedmark Rapport nr. 7 - 2001 Online-versjon Utgivelsessted: Elverum Det må ikke kopieres fra rapporten i strid med åndsverkloven og fotografiloven eller i strid med avtaler om kopiering inngått med KOPINOR, interesseorgan for rettighetshavere til åndsverk. Forfatteren er selv ansvarlig for sine konklusjoner. Innholdet gir derfor ikke nødvendigvis uttrykk for Høgskolens syn. I rapportserien fra Høgskolen i Hedmark publiseres FoU-arbeid og utredninger. Dette omfatter kvalifiseringsarbeid, stoff av lokal og nasjonal interesse, oppdragsvirksomhet, foreløpig publisering før publisering i et vitenskapelig tidsskrift etc. Rapporten kan bestilles ved henvendelse til Høgskolen i Hedmark. (http://www.hihm.no/Publikasjon/default.htm) Rapport nr. 7 - 2001 © Forfatteren/Høgskolen i Hedmark ISBN: 82-7671-167-7 ISSN: 1501-8563 2 Title: Cash, crops and cattle. A study of rural livelihoods in Botswana Author: Gerd Wikan Number: 7 Year: 2001 Pages: 182 ISBN: 82-7671-167-7 ISSN: 1501-8563 Financed by: Hedmark College and The research council of Norway Keywords: Botswana, livelihoods, rural poverty, income strategy Summary: Lack of economic development has lead to a growing scepticism to grand economic development theories and strategies. The focus has shifted towards a more open-ended perspective where the local context and poverty alleviation are in focus. As a result, the new key concepts in the discourse are livelihoods and urban-rural linkages. The academic interest is focused on the question: how are African households surviving given their increasing difficult economic circumstances? In the African context, Botswana is a special case.