Development of a Serological Test for Haemophilus Ducreyi For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reporting of Diseases and Conditions Regulation, Amendment, M.R. 289/2014
THE PUBLIC HEALTH ACT LOI SUR LA SANTÉ PUBLIQUE (C.C.S.M. c. P210) (c. P210 de la C.P.L.M.) Reporting of Diseases and Conditions Règlement modifiant le Règlement sur la Regulation, amendment déclaration de maladies et d'affections Regulation 289/2014 Règlement 289/2014 Registered December 23, 2014 Date d'enregistrement : le 23 décembre 2014 Manitoba Regulation 37/2009 amended Modification du R.M. 37/2009 1 The Reporting of Diseases and 1 Le présent règlement modifie le Conditions Regulation , Manitoba Règlement sur la déclaration de maladies et Regulation 37/2009, is amended by this d'affections , R.M. 37/2009. regulation. 2 Schedules A and B are replaced with 2 Les annexes A et B sont remplacées Schedules A and B to this regulation. par les annexes A et B du présent règlement. Coming into force Entrée en vigueur 3 This regulation comes into force on 3 Le présent règlement entre en vigueur January 1, 2015, or on the day it is registered le 1 er janvier 2015 ou à la date de son under The Statutes and Regulations Act , enregistrement en vertu de Loi sur les textes whichever is later. législatifs et réglementaires , si cette date est postérieure. December 19, 2014 Minister of Health/La ministre de la Santé, 19 décembre 2014 Sharon Blady 1 SCHEDULE A (Section 1) 1 The following diseases are diseases requiring contact notification in accordance with the disease-specific protocol. Common name Scientific or technical name of disease or its infectious agent Chancroid Haemophilus ducreyi Chlamydia Chlamydia trachomatis (including Lymphogranuloma venereum (LGV) serovars) Gonorrhea Neisseria gonorrhoeae HIV Human immunodeficiency virus Syphilis Treponema pallidum subspecies pallidum Tuberculosis Mycobacterium tuberculosis Mycobacterium africanum Mycobacterium canetti Mycobacterium caprae Mycobacterium microti Mycobacterium pinnipedii Mycobacterium bovis (excluding M. -

12. What's Really New in Antibiotic Therapy Print
What’s really new in antibiotic therapy? Martin J. Hug Freiburg University Medical Center EAHP Academy Seminars 20-21 September 2019 Newsweek, May 24-31 2019 Disclosures There are no conflicts of interest to declare EAHP Academy Seminars 20-21 September 2019 Antiinfectives and Resistance EAHP Academy Seminars 20-21 September 2019 Resistance of Klebsiella pneumoniae to Pip.-Taz. olates) EAHP Academy Seminars 20-21 September 2019 https://resistancemap.cddep.org/AntibioticResistance.php Multiresistant Pseudomonas Aeruginosa Combined resistance against at least three different types of antibiotics, 2017 EAHP Academy Seminars 20-21 September 2019 https://atlas.ecdc.europa.eu/public/index.aspx Distribution of ESBL producing Enterobacteriaceae EAHP Academy Seminars 20-21 September 2019 Rossolini GM. Global threat of Gram-negative antimicrobial resistance. 27th ECCMID, Vienna, 2017, IS07 Priority Pathogens Defined by the World Health Organisation Critical Priority High Priority Medium Priority Acinetobacter baumanii Enterococcus faecium Streptococcus pneumoniae carbapenem-resistant vancomycin-resistant penicillin-non-susceptible Pseudomonas aeruginosa Helicobacter pylori Haemophilus influenzae carbapenem-resistant clarithromycin-resistant ampicillin-resistant Enterobacteriaceae Salmonella species Shigella species carbapenem-resistant fluoroquinolone-resistant fluoroquinolone-resistant Staphylococcus aureus vancomycin or methicillin -resistant Campylobacter species fluoroquinolone-resistant Neisseria gonorrhoae 3rd gen. cephalosporin-resistant -

Reportable Disease Surveillance in Virginia, 2013
Reportable Disease Surveillance in Virginia, 2013 Marissa J. Levine, MD, MPH State Health Commissioner Report Production Team: Division of Surveillance and Investigation, Division of Disease Prevention, Division of Environmental Epidemiology, and Division of Immunization Virginia Department of Health Post Office Box 2448 Richmond, Virginia 23218 www.vdh.virginia.gov ACKNOWLEDGEMENT In addition to the employees of the work units listed below, the Office of Epidemiology would like to acknowledge the contributions of all those engaged in disease surveillance and control activities across the state throughout the year. We appreciate the commitment to public health of all epidemiology staff in local and district health departments and the Regional and Central Offices, as well as the conscientious work of nurses, environmental health specialists, infection preventionists, physicians, laboratory staff, and administrators. These persons report or manage disease surveillance data on an ongoing basis and diligently strive to control morbidity in Virginia. This report would not be possible without the efforts of all those who collect and follow up on morbidity reports. Divisions in the Virginia Department of Health Office of Epidemiology Disease Prevention Telephone: 804-864-7964 Environmental Epidemiology Telephone: 804-864-8182 Immunization Telephone: 804-864-8055 Surveillance and Investigation Telephone: 804-864-8141 TABLE OF CONTENTS INTRODUCTION Introduction ......................................................................................................................................1 -

Marion County Reportable Disease and Condition Summary, 2015
Marion County Reportable Disease and Condition Summary, 2015 Marion County Health Department 3180 Center St NE, Salem, OR 97301 503-588-5357 http://www.co.marion.or.us/HLT Reportable Diseases and Conditions in Marion County, 2015 # of Disease/Condition cases •This table shows all reportable Chlamydia 1711 Animal Bites 663 cases of disease, infection, Hepatitis C (chronic) 471 microorganism, and conditions Gonorrhea 251 Campylobacteriosis 68 in Marion County in 2015. Latent Tuberculosis 68 Syphilis 66 Pertussis 64 •The 3 most reported Salmonellosis 52 E. Coli 31 diseases/conditions in Marion HIV Infection 20 County in 2015 were Chlamydia, Hepatitis B (chronic) 18 Elevated Blood Lead Levels 17 Animal Bites, and Chronic Giardia 14 Pelvic Inflammatory Disease 13 Hepatitis C. Cryptosporidiosis 11 Cryptococcus 9 Carbapenem-resistant Enterobacteriaceae 8 •Health care providers report all Haemophilus Influenzae 8 Tuberculosis 6 cases or possible cases of Shigellosis 3 diseases, infections, Hepatitis C (acute) 2 Listeriosis 2 microorganisms and conditions Non-TB Mycobacteria 2 within certain time frames as Rabies (animal) 2 Scombroid 2 specified by the state health Taeniasis/Cysticercosis 2 Coccidioidomycosis 1 department, Oregon Health Dengue 1 Authority. Hepatitis A 1 Hepatitis B (acute) 1 Hemolytic Uremic Syndrome 1 Legionellosis 1 •A full list of Oregon reportable Malaria 1 diseases and conditions are Meningococcal Disease 1 Tularemia 1 available here Vibriosis 1 Yersiniosis 1 Total 3,595 Campylobacter (Campy) -Campylobacteriosis is an infectious illness caused by a bacteria. -Most ill people have diarrhea, cramping, stomach pain, and fever within 2-5 days after bacteria exposure. People are usually sick for about a week. -

Francisella Tularensis 6/06 Tularemia Is a Commonly Acquired Laboratory Colony Morphology Infection; All Work on Suspect F
Francisella tularensis 6/06 Tularemia is a commonly acquired laboratory Colony Morphology infection; all work on suspect F. tularensis cultures .Aerobic, fastidious, requires cysteine for growth should be performed at minimum under BSL2 .Grows poorly on Blood Agar (BA) conditions with BSL3 practices. .Chocolate Agar (CA): tiny, grey-white, opaque A colonies, 1-2 mm ≥48hr B .Cysteine Heart Agar (CHA): greenish-blue colonies, 2-4 mm ≥48h .Colonies are butyrous and smooth Gram Stain .Tiny, 0.2–0.7 μm pleomorphic, poorly stained gram-negative coccobacilli .Mostly single cells Growth on BA (A) 48 h, (B) 72 h Biochemical/Test Reactions .Oxidase: Negative A B .Catalase: Weak positive .Urease: Negative Additional Information .Can be misidentified as: Haemophilus influenzae, Actinobacillus spp. by automated ID systems .Infective Dose: 10 colony forming units Biosafety Level 3 agent (once Francisella tularensis is . Growth on CA (A) 48 h, (B) 72 h suspected, work should only be done in a certified Class II Biosafety Cabinet) .Transmission: Inhalation, insect bite, contact with tissues or bodily fluids of infected animals .Contagious: No Acceptable Specimen Types .Tissue biopsy .Whole blood: 5-10 ml blood in EDTA, and/or Inoculated blood culture bottle Swab of lesion in transport media . Gram stain Sentinel Laboratory Rule-Out of Francisella tularensis Oxidase Little to no growth on BA >48 h Small, grey-white opaque colonies on CA after ≥48 h at 35/37ºC Positive Weak Negative Positive Catalase Tiny, pleomorphic, faintly stained, gram-negative coccobacilli (red, round, and random) Perform all additional work in a certified Class II Positive Biosafety Cabinet Weak Negative Positive *Oxidase: Negative Urease *Catalase: Weak positive *Urease: Negative *Oxidase, Catalase, and Urease: Appearances of test results are not agent-specific. -

Haemophilus Influenzae Invasive Disease ! Report Immediately 24/7 by Phone Upon Initial Suspicion Or Laboratory Test Order
Haemophilus Influenzae Invasive Disease ! Report immediately 24/7 by phone upon initial suspicion or laboratory test order PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report for people <5 years old Review background on disease (see page 2), case definition (see page 4), and laboratory testing (see page 5) For cases in people ≥5 years old, interviews/investigations are not recommended unless the illness is known to be caused by H. influenzae type B. Surveillance for H. influenzae invasive disease in people ≥5 years old is now conducted only through electronic laboratory reporting (ELR) surveillance Contact health care provider to obtain pertinent information including demographics, medical records, vaccination history, and laboratory results Facilitate serotyping of H. influenzae isolates for people <5 years old at Florida Bureau of Public Health Laboratories (BPHL) Jacksonville Determine if the isolate is H. influenzae type b (Hib) Interview patient’s family or guardian Review disease facts Modes of transmission Incubation period Symptoms/types of infection Ask about exposure to relevant risk factors Exposure to a person with documented H. influenzae infection H. influenzae type B vaccination history Patient with immunocompromised state – HIV, sickle cell, asplenia, malignancy Determine if patient was hospitalized for reported illness Document pertinent clinical symptoms and type of infection Document close contacts (see page 7) and family members who may be at risk if Hib is identified Determine whether patient or symptomatic contact is in a sensitive situation (daycare or other settings with infants or unvaccinated children) Recommend exclusion for patients or symptomatic contacts until 24 hours of effective antibiotic treatment. -

Haemophilus Influenzae Disease: Commonly Asked Questions
Minnesota Department of Health Fact Sheet 1/2009 Haemophilus Influenzae Disease: Commonly Asked Questions What is Haemophilus influenzae? How is Haemophilus influenzae diagnosed? Haemophilus influenzae is a bacteria that is found in the nose and throat of children and Haemophilus influenzae is diagnosed adults. Some people can carry the bacteria in when the bacteria are grown from cultures their bodies but do not become ill. of the blood, cerebral spinal fluid (CSF) or other normally sterile body site. Cultures Haemophilus influenzae serotype B (Hib) is take a few days to grow. commonly associated with infants and young children and was once the most common cause of severe bacterial infection in children. How is Haemophilus influenzae Due to widespread use of Hib vaccine in infection treated? children, few cases are reported each year. Serious infections are treated with specific Non-serotype B infections occur primarily antibiotics. among the elderly and adults with underlying disease. There are no vaccines available against non-serotype B disease. Should people who have been in contact with someone diagnosed with Haemophilus influenzae be treated? What are the symptoms of Haemophilus influenzae? For Hib disease, treatment with specific antibiotics is recommended for household Haemophilus influenzae causes a variety of members when there is at least one illnesses including meningitis (inflammation unvaccinated child under 4 years of age in of the coverings of the spinal column and the home. Preventive treatment for non- brain), bacteremia (infection of the blood), vaccinated daycare center contacts of pneumonia (infection of the lungs), and known Hib cases may also be septic arthritis (infection of the joints). -
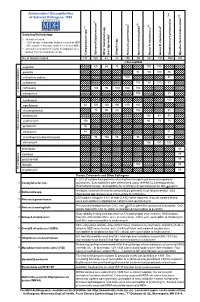
Antimicrobial Susceptibilities of Selected Pathogens, 1999
✔ Antimicrobial Susceptibilities * * † 7 of Selected Pathogens, 1999 8 e † a 2 ✔ ✔ † 3 ✔ † 4 5 6 culosis * r 1 urium spp. m L spp. Sampling Methodology 2 L † all isolates tested * ~ 20% sample of statewide isolates received at MDH spp. ~10% sample of statewide isolates received at MDH Salmonella ** all isolates tested from 7-county metropolitan area oup A streptococci ✔ oup B streptococci r isolates from a normally sterile site r Other (non-typhoidal) G Campylobacter Salmonella typhi Shigella Neisseria gonorrhoeae Neisseria meningitidis G Streptococcus pneumoni Mycobacterium tube No. of Isolates Tested 131 160 43 20 250 55 162 192 559 163 123456 123456% Susceptib123456le 123456123456 123456 123451623456 123451623456 ampicillin 1234561234566012345686 123456 15123456123456 100 100 123456123456 123451623451623451623451623456 123456 penicillin 123456123456123456123456123456 98100 100 76 123456 123456123456123456123456123456123456123456123456 123456 123451623451623451623456 123451623451623456 123456 cefuroxime sodium 123456123456123456123456 100123456123456123456 81 123456 123456123456123456123456123456123456123456123456 123456 cefotaxime 123451623451623451623451623456 100 100100 83 123456 123456123456123456123456123456 123456123456123456123456 123456 123456123456123456123456 ceftriaxone 123456 100 95 100 100 100 123451623451623451623456 -lactam antibiotics 123456123456123456123456123456 123456123456123456123456 β 123451623451623451623451623456 123451623456 123456 meropenem 123456123456123456123456123456 100 123456123456 83 123456 123456123456123456123456123456123456123456123456 -

Microbial NAD Metabolism: Lessons from Comparative Genomics
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 9-2009 Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga Rebecca Stebbins Sheila Z. Chang Mark A. McPeek Dartmouth College Charles Brenner Carver College of Medicine Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Biochemistry Commons, Genetics and Genomics Commons, Medicine and Health Sciences Commons, and the Microbiology Commons Dartmouth Digital Commons Citation Gazzaniga, Francesca; Stebbins, Rebecca; Chang, Sheila Z.; McPeek, Mark A.; and Brenner, Charles, "Microbial NAD Metabolism: Lessons from Comparative Genomics" (2009). Dartmouth Scholarship. 1191. https://digitalcommons.dartmouth.edu/facoa/1191 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Sept. 2009, p. 529–541 Vol. 73, No. 3 1092-2172/09/$08.00ϩ0 doi:10.1128/MMBR.00042-08 Copyright © 2009, American Society for Microbiology. All Rights Reserved. Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga,1,2 Rebecca Stebbins,1,2 Sheila Z. Chang,1,2 Mark A. McPeek,2 and Charles Brenner1,3* Departments of Genetics and Biochemistry and Norris Cotton Cancer Center, Dartmouth Medical School, Lebanon, New Hampshire -

The Old Testament Is Dying a Diagnosis and Recommended Treatment 1St Edition Download Free
THE OLD TESTAMENT IS DYING A DIAGNOSIS AND RECOMMENDED TREATMENT 1ST EDITION DOWNLOAD FREE Brent A Strawn | 9780801048883 | | | | | David T. Lamb Strawn offers a few other concrete suggestions about how to save the Old Testament, illustrating several of these by looking at the book of Deuteronomy as a model for second language acquisition. Retrieved 27 August The United States' Centers for Disease Control and Prevention CDC currently recommend that individuals who have been diagnosed and treated for gonorrhea avoid sexual contact with others until at least one week past the final day of treatment in order to prevent the spread of the bacterium. Brent Strawn reminds us of the Old Testament's important role in Christian faith and practice, criticizes current misunderstandings that contribute to its neglect, and offers ways to revitalize its use in the church. None, burning with urinationvaginal dischargedischarge from the penispelvic paintesticular pain [1]. Stunted language learners either: leave faith behind altogether; remain Christian, but look to other resources for how to live their lives; or balkanize in communities that prefer to speak something akin to baby talk — a pidgin-like form of the Old Testament and Bible as a whole — or, worse still, some sort of creole. Geoff, thanks for the reference. Log in. The guest easily identified the passage The Old Testament Is Dying A Diagnosis and Recommended Treatment 1st edition the New Testament, but the Old Testament passage was a swing, and a miss. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. -

Virulence Genes and Prevention of Haemophilus Influenzae Infections
Arch Dis Child: first published as 10.1136/adc.60.12.1193 on 1 December 1985. Downloaded from Archives of Disease in Childhood, 1985, 60, 1193-1196 Current topic Virulence genes and prevention of Haemophilus influenzae infections E R MOXON Infectious Disease Unit, Department of Paediatrics, John Radcliffe Hospital, Oxford The bacterium Haemophilus influenzae causes a infections) are encapsulated.3 H influenzae may wide spectrum of important childhood diseases that make any one of six chemically and antigenically includes meningitis, epiglottitis, cellulitis, acute distinct polysaccharide capsules (designated a-f), pneumonitis, septic arthritis, and otitis media. but strains expressing type b antigen account for Meningitis, the commonest of the systemic infec- most serious infections. The second important tions, in addition to being life threatening, is of observation was that serum factors (later identified particular importance to paediatricians because the as antibodies) with specific activity against the type damage it causes to the developing brain is often b antigen are critical in host defence against systemic permanent. H influenzae is a major cause of H influenzae infections.4 Given these facts, it is pyogenic meningitis in childhood throughout the reasonable to ask what is so important about the how does it differ world, and occurs in about one child in every type b capsule of H influenzae, copyright. thousand, usually within three years of birth. from the five other polysaccharide capsules, and to Although the availability of antibiotics has de- what extent other surface antigens, such as outer creased mortality dramatically (from greater than membrane proteins and lipopolysaccharide, modu- 90% to less than 10%), the occurrence of central late H influenzae virulence or serve as targets for the nervous system damage among survivors has not lethal effects of host immune responses. -

The Global View of Campylobacteriosis
FOOD SAFETY THE GLOBAL VIEW OF CAMPYLOBACTERIOSIS REPORT OF AN EXPERT CONSULTATION UTRECHT, NETHERLANDS, 9-11 JULY 2012 THE GLOBAL VIEW OF CAMPYLOBACTERIOSIS IN COLLABORATION WITH Food and Agriculture of the United Nations THE GLOBAL VIEW OF CAMPYLOBACTERIOSIS REPORT OF EXPERT CONSULTATION UTRECHT, NETHERLANDS, 9-11 JULY 2012 IN COLLABORATION WITH Food and Agriculture of the United Nations The global view of campylobacteriosis: report of an expert consultation, Utrecht, Netherlands, 9-11 July 2012. 1. Campylobacter. 2. Campylobacter infections – epidemiology. 3. Campylobacter infections – prevention and control. 4. Cost of illness I.World Health Organization. II.Food and Agriculture Organization of the United Nations. III.World Organisation for Animal Health. ISBN 978 92 4 156460 1 _____________________________________________________ (NLM classification: WF 220) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO web site (www.who.int/about/licensing/copyright_form/en/index. html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.