Acme (Itter 9Das& Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taipei Times Article
FEATURES SUNDAY, SEPTEMBER 4, 2016 Master potter Wu Yao- cheng brought the YINGGE TOWN eggshell porcelain P8 trimming skills he Artisan P8 learned as a young boy to Taiwan with Yingge Town Artisan is a Editorial monthly photographic and him at the end of the historical exploration of the Editorial Chinese Civil War, artists and potters linked to and he’s still going New Taipei City’s Yingge Town. strong BY PauL COOper STAFF REPORTER Working on 紅 紅 藍 藍 黃 黃 黑 eggshells 黑 Yao-cheng (吳要城), 84, holds ing to Cheng Wen-hung (程文宏), head of father got work and accommodation in a lent technique. up a white porcelain bowl to the Educational Promotion Department local ceramics factory. Wang says that unlike other clays, Wu the window in his studio home of the New Taipei City Yingge Ceram- Wu started helping to produce ceram- porcelain clay has no “bone,” or structure, in Yingge District (鶯歌), New Taipei City. ics Museum, he was a pioneer of these ics at the age of 12. The factory produced making it very difficult to throw. On the inside, it is painted with auspi- techniques when he first arrived in functional wares, but some of the more But achieving the extreme thinness cious symbols; on the outside, there is a Yingge, and is still one of very few, maybe highly skilled workers were also making for eggshell pieces also requires manu- scene of monkeys. Wu didn’t paint it: his the only one, still capable of produc- imitations of sought-after Song, Ming ally trimming the unfired porcelain pieces specialty is making the eggshell porcelain ing eggshell porcelains with traditional and Qing dynasty ceramics, including with steel trimming blades, on a rapidly (薄胎瓷) bodies. -

Mozi Precision Co., Ltd
MOZi Precision Co., Ltd. Company Introduction 1 Company introduction Mozi Precision Co. Ltd, was established in 2006 with initial capital NTD$ 140 million dollars. Mozi is a 4000 square meters ISO 9001 factory, located at Yingge district, New Taipei City. Mozi currently has 60 employees and run a 24hrs operation. Mozi specializes at: Plastic precision mold Aluminum/zinc die-casting molds Plastic injection Metal milling machine (CNC) Plastic and metal coating/painting, silk screen and pad printing Mozi-Precision is the one stop solution provider. Fast and punctual delivery, short tooling development time, consistence of quality assurance forming the strongest fundamental for Mozi-Precision. Mozi offers most competitive price with excellent services. Mozi guarantees our customers with the best fulfillment and innovation. 2 Core Service Mold design and Injection / Die-Cast Manufacture Core Technology Metal milling and Surface Treatment CNC process 3 Business Contents Molds Plastic Injection Surface treatment • Die-Casting • ABS/PC • Metal Coating • Extrusion • PA/PC/PPS+GF% • Plastic Painting • Plastic precision • Highly polished • Texture look molds products Spray Painting • Unscrewing mold • Insert Molding • Anodize look • Special mold • Wrapping by Spray Painting injection • Surface Conversion Process • Sliver Chroming CNC Spray Painting • CNC Milling • PU Coating • CNC Tapping • Silk Screen Printing • 4-axis machining • Pad Printing • CNC Machining • Thermal transfer printing 4 Development Process of Molds Mold PO confirm 3D Drawing DFM Mold design Design for manufacturing Manufacturability Trial T2 review T1 review T2 production 1. FAI 1. FAI T1 2. Appearance 2. Appearance review review 3.10pcs samples 3. Trial fit of parts Trial production review Samples for Approval 1. -
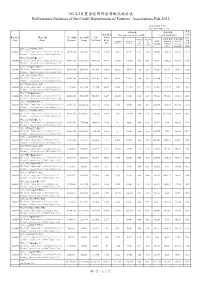
101年2月農會信用部經營概況統計表performance Statistics of The
101年2月農會信用部經營概況統計表 Performance Statistics of the Credit Departments of Farmers’ Associations,Feb-2012 單位:新臺幣 千元,% Unit:NT$1,000 percent 逾期放款 備抵呆帳 淨值 本期損益 Non-performing Loan(NPL) Loan Loss Deposit 佔風 單位代號 單位名稱 存款總額 放款總額 淨值 Profit 險性 占狹義逾期 占廣義逾期 Code Name Deposits Loans Net Worth Before 狹義逾期比 廣義逾期比 資產 狹義逾期放款 廣義逾期放款 率 率 金額 放款比率 放款比率 Tax NPL(Old) NPL(New) NPL NPL Amount Coverage Coverage 比率 Ratio(Old) Ratio(New) Rate Rate(New) BIS 新北市三重區農會信用部 6060019 The Credit Department of Sanchong District 14,893,908 9,490,881 1,519,341 23,458 12,477 38,197 0.13 0.40 134,839 1,080.70 353.01 19.77 Farmers' Association of New Taipei City 新北市板橋區農會信用部 6060020 The Credit Department of Banciao District 40,671,274 29,031,313 4,933,179 34,179 23,354 122,042 0.08 0.42 529,331 2,266.55 433.73 23.54 Farmers' Association of New Taipei City 新北市淡水區農會信用部 6060031 The Credit Department of Danshuei District 10,796,680 5,599,994 591,469 6,839 68,242 165,673 1.22 2.96 97,289 142.56 58.72 12.19 Farmers' Association of New Taipei City 新北市樹林區農會信用部 6060042 The Credit Department of Shulin District 26,288,960 16,453,526 2,532,105 14,477 40,191 95,075 0.24 0.58 311,524 775.11 327.66 16.71 Farmers' Association of New Taipei City 新北市鶯歌區農會信用部 6060053 The Credit Department of Yingge District 9,775,066 6,133,704 973,784 10,077 6,858 112,537 0.11 1.83 89,002 1,297.78 79.09 15.02 Farmers' Association of New Taipei City 新北市三峽區農會信用部 6060064 The Credit Department of Sansia District 10,273,308 5,107,695 560,008 6,667 15,325 64,883 0.30 1.27 89,316 582.81 137.66 12.81 Farmers' Association -

Point Cloud-Oriented Inspection of Old Street's Sustainable Transformation from the Ceramic Industry to Cultural Tourism
sustainability Article Point Cloud-Oriented Inspection of Old Street’s Sustainable Transformation from the Ceramic Industry to Cultural Tourism: A Case Study of Yingge, a Ceramic Town in Taiwan Naai-Jung Shih * , Wen-Tse Hsu and Pei-Huang Diao Department of Architecture, National Taiwan University of Science and Technology, 43, Section 4, Keelung Road, Taipei 106, Taiwan * Correspondence: [email protected]; Tel.: +886-02-2737-6718 Received: 25 July 2019; Accepted: 28 August 2019; Published: 30 August 2019 Abstract: Yingge, a ceramic-producing town in Northern Taiwan, has experienced three development stages in the 50 years since 1970. The town’s fabric and the second contour evolved through the transformation of its former manufacturing industry into cultural tourism on Old Street. This process of evolution is evidenced through chronological changes of overlaid sections, skylines, and horizontal sections along Old Street since 1970. The street fabric has been shaped by its historical background, government planning strategies, commercial activities, cultural identity, and living patterns. Three-dimensional (3D) scans supported our analysis by capturing and segmenting the vocabularies and overlaid sections with special characteristics and changes. Commercial spaces and open street spaces were found to be mutually influential. A flexible and sometimes hidden spatial structure of fabric was elucidated. Yingge has become a large-scale shopping mall and important window into cultural tourism, with its fabric and contours redeveloped to be consistent with the identity of nearby cities. 3D scanning data were combined with documentation and maps to create a referable connection between reality and chronological data. An augmented reality (AR) application was used to simplify the inspection process through a productive connection between as-built scans and user interactions. -

Vertical Facility List
Facility List The Walt Disney Company is committed to fostering safe, inclusive and respectful workplaces wherever Disney-branded products are manufactured. Numerous measures in support of this commitment are in place, including increased transparency. To that end, we have published this list of the roughly 7,600 facilities in over 70 countries that manufacture Disney-branded products sold, distributed or used in our own retail businesses such as The Disney Stores and Theme Parks, as well as those used in our internal operations. Our goal in releasing this information is to foster collaboration with industry peers, governments, non- governmental organizations and others interested in improving working conditions. Under our International Labor Standards (ILS) Program, facilities that manufacture products or components incorporating Disney intellectual properties must be declared to Disney and receive prior authorization to manufacture. The list below includes the names and addresses of facilities disclosed to us by vendors under the requirements of Disney’s ILS Program for our vertical business, which includes our own retail businesses and internal operations. The list does not include the facilities used only by licensees of The Walt Disney Company or its affiliates that source, manufacture and sell consumer products by and through independent entities. Disney’s vertical business comprises a wide range of product categories including apparel, toys, electronics, food, home goods, personal care, books and others. As a result, the number of facilities involved in the production of Disney-branded products may be larger than for companies that operate in only one or a limited number of product categories. In addition, because we require vendors to disclose any facility where Disney intellectual property is present as part of the manufacturing process, the list includes facilities that may extend beyond finished goods manufacturers or final assembly locations. -

Eighty-Third Executive Committee
12-13 APRIL 2012 Report BUCHAREST, ROMANIA BUCHAREST, SESSION RD 83 FMD COMMISSION u OF THE E OF THE EXECUTIVE COMMITTEE BUCHAREST, ROMANIA 12-13 APRIL 2012 • 83RD SESSION OF THE EXECUTIVE COMMITTEE OF THE EuFMD COMMISSION • REPORT BUCHAREST, ROMANIA 12-13 APRIL 2012 Report 83RD SESSION OF THE EXECUTIVE COMMITTEE OF THE EuFMD COMMISSION FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS EUROPEAN COMMISSION FOR THE CONTROL OF FOOT-AND-MOUTH DISEASE (EuFMD) Rome, 2012 2 Table of Contents Conclusions and Recommendations ................................................................................................................ 4 Meeting Report ................................................................................................................................................ 7 1. Adoption of the Agenda. ....................................................................................................................... 8 2. Report on activities conducted under the framework of the EuFMD/EC Agreement .......................... 8 Report ....................................................................................................................................................... 8 FAO Evaluation options and process ........................................................................................................ 8 3. FMD epidemic situation in the region ................................................................................................... 9 Overview of FMD virus circulation and risk (WRL Report) -

To Meet Same-Day Flight Cut-Off Time and Cut-Off Time for Fax-In Customs Clearance Documents
Zip Same Day Flight No. Area Post Office Name Telephone Number Address Code Cut-off time* No. 114, Sec. 1, Jhongsiao W. Rd., Jhongjheng District, Taipei 100-12, Taiwan 1 Greater Taipei Taipei Beimen Post Office 100 (02)2361-5752(02)2381-3135 16:00 ( R.O.C.) 1F, No. 162, Sec. 1, Singsheng S. Rd., Jhongjheng District, Taipei 100-61, Taiwan 3 Greater Taipei Taipei Dongmen Post Office 100 (02)2394-7576(02)2321-4679 14:45 (R.O.C.) 4 Greater Taipei Taipei Nanhai Post Office 100 (02)2396-3142(02)2321-4189 No. 43, Sec. 2, Chongcing S. Rd., Jhongjheng District, Taipei 100-75, Taiwan (R.O.C.) 14:40 (02)2368-4714 5 Greater Taipei Taipei Yingciao Post Office 100 No. 76, Siamen St., Jhongjheng District, Taipei 100-83, Taiwan (R.O.C.) 14:50 (02)2367-0905 6 Greater Taipei Taipei Fusing BridgePost Office 100 (02)2311-4421 No. 1, Sec. 1, Jhongsiao W. Rd., Jhongjheng District, Taipei 100-41, Taiwan (R.O.C.) 15:35 7 Greater Taipei Executive Yuan Post Office 100 (02)2321-4874 No. 2, Beiping E. Rd., Jhongjheng District, Taipei 100-51, Taiwan (R.O.C.) 15:25 (02)2331-0962 8 Greater Taipei Academia Historica Post Office 100 No. 168-1, Bo-ai Rd., Jhongjheng District, Taipei 100-48, Taiwan (R.O.C.) 15:50 (02)2311-7879 No. 122, Sec. 1, Chongcing S. Rd., Jhongjheng District, Taipei 100-48, Taiwan 9 Greater Taipei Presidential Office Building Post Office 100 (02)2311-0859 D+1 (R.O.C.) (02)2331-4925 10 Greater Taipei Taipei District Court Post Office 100 No. -

Yingge District, New Taipei City, Chinese Taipei
THE INTERNATIONAL AWARDS FOR LIVABLE COMMUNITIES 2012 WHOLE CITY SUBMISSION CATEGORY C Taiwan’s Ceramic Art Town on the Bank of Dahan Stream Yingge District, New Taipei City Taiwan’s Ceramic Art Town on the Bank of Dahan Stream-Yingge District Taiwan’s Ceramic Art Town on the Bank of Dahan Stream- Yingge District, New Taipei City Overview Yingge District is located in the most peripheral part of the New Taipei City. Both Yingge and Sanxia are important gateways into the New Taipei City on Dahan Stream. It faces Linkou Plateau on the north and its land elevations slope from northeast to southwest. The district has a total area of 21.12 square kilometers (1.06% of New Taipei City) with less than a third of level ground. The population has increased by 6.3% within 10 years, with a total of 28,073 households (1.95% of New Taipei City). The total population is 87,710 (2.23% of New Taipei City) with a density of 4,155 people per square kilometer. For the transportation network, Taiwan Railway Western Line currently has Yingge Station and another future station Fengming in planning. The National Highway No. 3 is easily accessible at the nearby interchange. It is also very convenient to reach the National Highway No. 1, Taoyuan International Airport, and Taiwan High Speed Rail Taoyuan Station via Danan Interchange on the National Highway No. 2. Taoyuan County Shulin District Yingge District Sanxia District Dahan Stream Figure 1: Location Map of Yingge District 1 The International Awards for Livable Communities 2012-Category C Enhancement of the Natural and Built (A) Protection of the Spiritual Landmark - Yingge Rock Yingge Rock (Yingge is the Chinese pronunciation of parrot), a sedimentary rock formed over hundreds of years, is an important landmark in the district. -

Gary Li Peter W. Fritsch
A TAXONOMIC REVISION OF TAXA IN STYRAX SERIES CYRTA (STYRACACEAE) WITH VALVATE COROLLAS Gary Li Peter W. Fritsch Department of Botany Botanical Research Institute of Texas California Academy of Sciences 1700 University Drive 55 Music Concourse Drive Fort Worth, Texas 76107, U.S.A. San Francisco, California 94118, U.S.A. [email protected] Department of Biology, San Francisco State University 1600 Holloway Avenue, San Francisco, California 94132, U.S.A. ABSTRACT Several taxonomic treatments of Styrax (Styracaceae) exist in regional floras of Asia, but the Asian species of the genus have not been com- prehensively revised since 1907. A treatment of the Asian taxa of S. series Cyrta with imbricate floral aestivation was accomplished in 2003. To complete the taxonomic revision of S. series Cyrta, we conducted a taxonomic revision of the species of the series with valvate aestivation of the corolla lobes. Our revision comprises 11 species with a combined distribution from eastern India through southern China and Malesia, Melanesia, and Micronesia, although the group is absent from the Philippines. We resurrected S. bracteolatus, S. rubifolius, and S. warburgii as species to be recognized, and we corrected the misapplication of S. finlaysonianus, previously used for a species in S. series Benzoin. Styrax finlaysonianus and S. warburgii are segregated from the broader concept of S. agrestis recognized in prior treatments. The circumscriptions of the heretofore poorly delimited species S. confusus, S. faberi, and S. fortunei are clarified and their possible introgressants discussed. We observed unique combinations of characters in some problematic specimens whose taxonomic status remains unresolved because only single specimens with either flowers or fruits were available; at least some of these may represent undescribed species. -

New Taipei City Government
Environmental Protection Department New Taipei City Government ClimateNew Taipei Adaptation City Action Plan (Abridged Edition) HAZARD RISK CLIMATE CHANGE ADAPTATION August 2015 I hereby declare the intent of the city of New Taipei to comply with the Com- pact of Mayors, the world’s largest cooperative effort among mayors and city leaders to reduce greenhouse gas emissions, track progress, and prepare for the impacts of climate change. The Compact of Mayors has defined a series of requirements that cities are expected to meet over time, recognizing that each city may be at a differ- ent stage of development on the pathway to compliance with the Com- pact. I commit to advancing the city of New Taipei along the stages of the Com- pact, with the goal of becoming fully compliant with all the requirements within three years. Specifically, I pledge to publicly report on the following within the next three years: •The greenhouse gas emissions inventory for our city consistent with the Global Protocol for Community-Scale Greenhouse Gas Emission Inventories (GPC), within one year or less •The climate hazards faced by our city, within one year or less •Our target to reduce greenhouse gas emissions, within two years or less •The climate vulnerabilities faced by our citiy, within two years or less •Our plans to address climate change mitigation and adaptation within three years of less Yours Faithfully, ■ CONTENTS CHAPTER 1 /Origin and Purpose1 CHAPTER 2 Hazard Identification 2 /New Taipei City Environmental Status 2 Hazard Identification Techniques -

Annual Report Is Available At
Stock Code : 2542 Printed on April 13, 2020 The Annual Report is available at http://mops.twse.com.tw/ https://www.highwealth.com.tw/ Dream City, Taichung HighwealthHighwealth CConstructiononstruction CorporationCorporation 2019 Annual Report I.Spokesperson Deputy Spokesperson Name:Liao-Zhaoxiong Name:Wang-Suyue Title:Development Dept. Vice President Title:Finance Dept. Vice President Tel:(02)2755-5899 Tel:(02)2755-5899 E-mail:[email protected] E-mail:[email protected] II.Headquarters and Branches Headquarters Address:10F., No.267, Lequn 2nd Rd., Zhongshan Dist., Taipei Tel:(02)2755-5899 Taichung Branches Address:25F., No.213, Chaofu Rd., Xitun Dist., Taichung Tel:(04)2252-6886 Kaohsiung Branches Address:19F., No.1507-1, Yucheng Rd., Gushan Dist., Kaohsiung Tel:(07)552-6199 III.Stock Transfer Agent Name:Registrar Agency Department of Capital Securities Corporation Address:B2, No.97, Sec.2, Dunhua S. Rd., Da’an Dist., Taipei Tel:(02)2702-5000 Website:http://www.capital.com.tw/agency/ IV.Auditors in the most recent year KPMG Auditors:Jian-Dinuan;Zeng-Guoyang Address:68F, Taipei 101 Tower, No. 7, Sec. 5, Xinyi Road, Taipei Tel:(02)8101-6666 Website:http://www.kpmg.com.tw V.Overseas Securities Exchange N/A VI.Corporate Webisite http://www.highwealth.com.tw Table of Contents Page I. Letter to the Shareholders ............................................................................................................. 1 II.Company Profile ........................................................................................................................... -
City Tours Taipei, New Taipei/Taoyuan, Taichung, Tainan, and Kaohsiung by Public Transport and Public Rental Bike Introduction
Travel in TAIWAN Theme Guide City Tours Taipei, New Taipei/Taoyuan, Taichung, Tainan, and Kaohsiung by Public Transport and Public Rental Bike Introduction During the years of the Taiwan Economic Miracle in the last century, emphasis was placed on rapid material growth, with limited thought given to a city’s visual aesthetics. New times bring new thinking. In this century’s Taiwan, wealthy and ever more genteel, city populations have embraced the internationalist “livable city” concept with great passion. Innovative, cutting-edge green architecture is sprouting up like a beautifying forest. On the health front, healthy lifestyles with plenty of vigorous outdoor exercise is the new clarion call. A dense network of dedicated bike paths through green spaces and dedicated bike lanes on urban roads has been built up, with public bike-rental facilities always readily available, your high-quality self-powered mechanical steed made available at ultra-low cost. Adding to the convenience is the fact that bikes can be picked up at one rental station and dropped off at another, and that the stations are interlinked with other public-transport systems. In this booklet we showcase the wonderful experiences you can have in Taiwan’s biggest cities, which are lined up like a string of pearls from the island’s far north to far south along its west side. Each has a strikingly different personality. Fast-paced Taipei, which sits in a basin surrounded by mountains, is the political, financial, and cultural capital. The New Taipei/Taoyuan agglomeration provides ready seaside and low-mountain access. Light- industry Taichung is rich in wide roads and open spaces.