The Impact of the 2019/20 Fires on Platypus Populations in the Midcoast Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government Gazette No 184 of Thursday 19 December 2019
GOVERNMENT GAZETTE – 19 December 2019 Government Gazette of the State of New South Wales Number 184 Thursday, 19 December 2019 The New South Wales Government Gazette is the permanent public record of official NSW Government notices. It also contains local council, private and other notices. From 1 January 2019, each notice in the Government Gazette has a unique identifier that appears in round brackets at the end of the notice and that can be used as a reference for that notice (for example, (n2019-14)). The Gazette is compiled by the Parliamentary Counsel’s Office and published on the NSW legislation website (www.legislation.nsw.gov.au) under the authority of the NSW Government. The website contains a permanent archive of past Gazettes. To submit a notice for gazettal – see Gazette Information. By Authority ISSN 2201-7534 Government Printer NSW Government Gazette No 184 of 19 December 2019 pages 6313 to 6326 Temporary Water Restriction (Hastings Unregulated and Alluvial Water Sources) Order 2019 under the Water Management Act 2000 I, Allan Raine, by delegation from the Minister administering the Water Management Act 2000, in pursuance of section 324 (1) of the Water Management Act 2000 and being satisfied that it is necessary in the public interest to do so, make the following Order. Dated 20 December 2019 ALLAN RAINE A/Director, Water Planning Implementation Department of Planning, Industry and Environment By delegation Explanatory note The objects of this Order are as follows: (a) to impose temporary water restrictions on certain take of water from the Hastings Unregulated and Alluvial Water Sources by imposing conditions on when take is permitted and cease to pump restrictions, (b) to require the recording of take in logbooks for take from the Hastings Unregulated and Alluvial Water Sources and the Hastings River Coastal Floodplain Alluvial Groundwater. -

Water Quality in the Manning River Estuary Is Made by Determining to What Extend These Long-Term Goals Were Being Met
I ~ I i.; I ! I I Ii Ii GRE~~TER Ti\REE I II II:1 d CITY COlJNCIL !I I :! I I -'" ... ,,... .. , ... I I I I I I I- I I WATER QUAllIT IN THE MANNING RIVER . I 1989 - 94 I I 1- I I- I I I I I -I ~_ ... ~ __•. .(-."._.,~ .............. -.--=r ,.---' _. I I I I I I I I~ a report prepared by I Anna Kaliska I SEWER & WASTE SERVICES CRE_ATER TAREE CITY COUNCIL 2 Palteney Street I: TAREE NSW 2430 . Phone (06S) 913 399 I, October 1994 I !::", I I I I I I 1 INTRODUCTION I 2 MONITORING PROGRAM I 2.1 Stations Location 2.2 Flow Conditions During Sample Collection '1 ····2.3 Parameters-Neasl:<.reci·- . -- .,. .... .......-;:;..:;._._... --. -:::;;....: .. -' I 3 TUE MANNING RIVER SYSTEM 3.1 Catchment Description I 3.2 Tidal Behaviour and Sediment Transport 3.3 Water Resources and the River Flow I 4 TUE SOURCES OF WATER POLLUTION I 4.1 Point Source Discharges 4.2 Diffuse Source Pollution I I 5 WATER QUALITY IN TUE MANNING RIVER ESTUARY 5.1 Salinity 5.2 Dissolved Oxygen I 5.3 Biochemical Oxygen Demand 5.4 Clarity 5.5 Bacteriological Characteristics I 5.6 Nutrients I 6 CONCLUSIONS I REFERENCES I I 'I I TABLES 1 1 Water Quality Sampling Stations 2 Manning River Flow at Killawarra Station I 3 Estimated Manning River Flow at Taree 1 4 Characteristics of Sewage Treatment Plant Effluent I FIGURES I I 1 Location of Sample Collection Sites 2 Tidal Characteristics of the Manning River Estuary I 3 Load Discharged to the Manning River Before and after Taree Sewage Treatment Plant Flow Diversion I 4 Salinity - Mean Values 1989-94 1 5 Surface Dissolved Oxygen 1989-94 6 Bottom Dissolved Oxygen 1989-94 I 7 Average Dissolved Oxygen Ratios 1986, I 91/92 and 93/94 8 Biochemical Oxygen Demand 1989-94 I 9 Secchi Disc 1989-94 I 10 Faecal Coliforms 1989-94 11 Faecal Coliform 1989/92 and 93/94 I 12 Total Phosphorus 1989-94 ,-'Jo I, 1,3 Total Nitrogen 1989-94 14 Total Phosphorus 1984-94 15 Total Nitrogen 1984-94 = I I 1 1 INTRODUCTION I The Manning River system is one of the major river systems in New South Wales and is seen as an important natural resource on a local, regional and state level. -
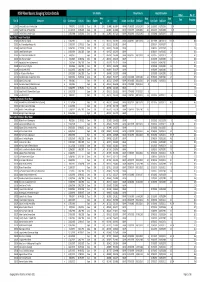
Gauging Station Index
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

The History of the Worimi People by Mick Leon
The History of the Worimi People By Mick Leon The Tobwabba story is really the story of the original Worimi people from the Great Lakes region of coastal New South Wales, Australia. Before contact with settlers, their people extended from Port Stephens in the south to Forster/Tuncurry in the north and as far west as Gloucester. The Worimi is made up of several tribes; Buraigal, Gamipingal and the Garawerrigal. The people of the Wallis Lake area, called Wallamba, had one central campsite which is now known as Coomba Park. Their descendants, still living today, used this campsite 'til 1843. The Wallamba had possibly up to 500 members before white contact was made. The middens around the Wallis Lake area suggest that food from the lake and sea was abundant, as well as wallabies, kangaroos, echidnas, waterfowl and fruit bats. Fire was an important feature of life, both for campsites and the periodic 'burning ' of the land. The people now number less than 200 and from these families, in the main, come the Tobwabba artists. In their work, they express images of their environment, their spiritual beliefs and the life of their ancestors. The name Tobwabba means 'a place of clay' and refers to a hill on which the descendants of the Wallamba now have their homes. They make up a 'mission' called Cabarita with their own Land Council to administer their affairs. Aboriginal History of the Great Lakes District The following extract is provided courtesy of Great Lakes Council (Narelle Marr, 1997): In 1788 there were about 300,000 Aborigines in Australia. -

(Phascolarctos Cinereus) on the North Coast of New South Wales
A Blueprint for a Comprehensive Reserve System for Koalas (Phascolarctos cinereus) on the North Coast of New South Wales Ashley Love (President, NPA Coffs Harbour Branch) & Dr. Oisín Sweeney (Science Officer, NPA NSW) April 2015 1 Acknowledgements This proposal incorporates material that has been the subject of years of work by various individuals and organisations on the NSW north coast, including the Bellengen Environment Centre; the Clarence Environment Centre; the Nambucca Valley Conservation Association Inc., the North Coast Environment Council and the North East Forest Alliance. 2 Traditional owners The NPA acknowledges the traditional Aboriginal owners and original custodians of the land mentioned in this proposal. The proposal seeks to protect country in the tribal lands of the Bundjalung, Gumbainggir, Dainggatti, Biripi and Worimi people. Citation This document should be cited as follows: Love, Ashley & Sweeney, Oisín F. 2015. A Blueprint for a comprehensive reserve system for koalas (Phascolarctos cinereus) on the North Coast of New South Wales. National Parks Association of New South Wales, Sydney. 3 Table of Contents Acknowledgements ....................................................................................................................................... 2 Traditional owners ........................................................................................................................................ 3 Citation ......................................................................................................................................................... -

Historical Riparian Vegetation Changes in Eastern NSW
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2016 Historical Riparian Vegetation Changes in Eastern NSW Angus Skorulis Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Skorulis, Angus, Historical Riparian Vegetation Changes in Eastern NSW, BSci Hons, School of Earth & Environmental Science, University of Wollongong, 2016. https://ro.uow.edu.au/thsci/120 Research Online is the open access institutional repository for the University of Wollongong. -

Water Sharing Plan for the Hastings Unregulated And
Locality Map ek re C s p o h s ek i re B C e r Stum ek y Kumba py C re p tine e C l re e e k T R e e d y C re e k G e a k ry Karst Cree C K o r i g k e Karst Gowings ll in e ek ic D e ek k Easy Creek r re Gowings Hill C C C C P r l a d e eg r len r Hill i e h G c a p orn Big a oe y e k s C H p C k r re il C p re c s ek l i e C a k o Karst C r K t k e s r e e c B k S eek Cre CRESCENT HEAD k a a e Gowings n d e o e n r i ! C d Hill t g a C c or e r C e G e r g C e e in n re k e s n ek k s Creek o ro ank C C eb Sm in iths T k Cree P e k k ie e Mo r e ek untai re Maria River re n C C ls C C r n el i e K ca w C o e o rri s o v k g o e Ca b k je Creek rlo M Water Source cro e n a fts e u M Cr r er eek C v C i O Forbes River n R 'L s so ea s Wil r o ek y re B C Goolawah Lagoon C r Water Source y e k e e n k e Pige e o o r n M C C y T o s b ad rry r o ra e Ba ive p b R R a Wilson River C l ia r d r e a e C M k r e e Water Source Big k Hill Cundle C C k r r e ee e e k Cr e ls Karst k l k e e B Big Hill B e r F r il C B o rabung Point rs k r k e Cr r ye ree i ree p eek b w C l C oo Sa sh C low C k e u s br ree ng e R tle k Ba e ot r iv e B C r e e D k tr ead M e Fe s Horse Creek ans Cre e e r nwicks ras k Creek G C Barneys Creek t le Creek In ders TELEGRAPH POINT R ud Saltwater Pappinbarra River ! Lake Water Source Upper Hastings River a C C oolapatamb re Limeburners ed eek C ek W ar d Cr at Creek Bitter Groun Co erfall wal Cre C Water Source ek r e e Creek k B re Mortons Creek alyngara C ek Water Kindee Creek Water Source reek Source M Loggy C -

Download the Site Map. PDF, 10720.16 KB
State forest Worimi cultural area Walking tracks Mountain Lookout Lookout Parking Toilets European history interpretive signage Playground Cli edge, no barrier fencing Supervise children Boolah-Dillah Track Mountain Track Mountain Ted Baker Track Track Boolah-Dillah Pacific Highway Roads You are here Bulahdelah Mountain Park Worimi Boolah-Dillah Cultural Track Area Ted Baker Bulahdelah Mountain Track An Aboriginal Place Ted Baker Lookout Bulahdelah Mountain is an Aboriginal Place in recognition of the cultural spiritual and historical significance of the area to the Worimi People. People talk about country in the same way they would talk about a person: they speak to 0 0.25 0.5 1 country, sing to country, visit country, worry about country, feel sorry for country and long Kilometers for country. People say that country knows, Explore hears, smells, takes notice, takes care, is sorry or Boolah Dillah Track Mountain Track Ted Baker Track happy…country is a living entity with a yesterday, From here to Worimi Cultural Area From Worimi Cultural Area to 840 m return, 20–40 min today and tomorrow, with a consciousness and a 2.2 km return, 40 min–1 hr Mountain Lookout Medium grade with many steps will towards life. The initial ascent is the steepest 1.7 km return, 30–45 min and level sections. The lookout Deborah Bird Rose ‘Nourishing Terrains’ 1996 and becomes moderate after the Medium grade with many steps and is natural rock cliff with no intersection with the Ted Baker Track. level sections. The lookout is natural barrier fencing. rock cliff with no barrier fencing. -

The Permanent Walk Booklet Update
1 2 THE OLD AUSTRALIAN AGRICULTURAL COMPANY KARUAH TO TAHLEE WALK BOOKLET (Revised for 2015) We acknowledge and recognise the Worimi people on whose land we walk. GENERAL INTRODUCTION WHY WALK? Once every year, Karuah residents and friends walk the 5 kilometres or so from Karuah to Tahlee along the Old AACo Road. It only happens once a year because the road crosses Yalimbah Creek and the bridge that used to cross the creek has gone. In the late 1950s, the bridge which had been built under the direction of Robert Dawson in 1826 was burnt down by persons unknown. At that stage, the bridge was more than 130 years old, a remarkable age for a wooden bridge. Up to that point residents of the two villages had travelled back and forth on a daily basis. From then on, they were forced to take the current route which is 14 kilometres long. So, every year for the last five years, a local oyster farmer has offered an oyster barge to carry people over the creek and around 150 people re-enact the trip from village to village. Karuah Progress association hosts the day which includes a light lunch, guides, afternoon tea and an inspection of historic Tahlee House and a bus ride back to Karuah via the new route as well as a photocopied version of this booklet. TAHLEE AND KARUAH – IN THE EARLY 19TH CENTURY: In 1825 when the Australian Agricultural Company was formed, 10,000 shares were offered at one hundred pounds per share and they were snapped up by the rich and famous. -

Midcoast Water
Who we are and what we do COMMUNITY INFORMATION BOOKLET 2016 Contents Introduction 3 MidCoast Water 4-5 Sustainable water cycle management 6 The water cycle 7 Our water supplies 8 The Manning Scheme 9-14 How does water get to our homes? 15 The treatment process 16-18 Other water supplies 19 Karuah River and Great Lakes Catchment 20 Water supply schemes 21-24 How much water do we use? 25 Let’s get waterwise 26 Don’t spray in the middle of the day! 27 Wastewater 28-31 Recycling 32 Wipes stop pipes 33 Think at the sink 34 Sewer spills 35 Water Quality Testing 36-37 Paying for it all 38-40 Does everyone have clean water? 41 For further information 42 2 Who we are and what we do Meet Whizzy: Introduction This is Whizzy the Waterdrop, MidCoast Water’s mascot. Whizzy Every day MidCoast Water cleans and pumps almost helps to remind us how 10 Olympic swimming pools worth of water through important it is to save a network of over a thousand kilometres of pipes to water and is a favourite of make sure that the people of the Manning, Great Lakes the children in our area. and Gloucester have ready access to safe water for all For more information on Whizzy email their needs. That water is used by almost 80 000 people community@ in 27 towns from Crowdy Head in the north, to Hawks midcoastwater.com.au Nest in the south, and Barrington in the west, before we take and treat the waste. -

Government Gazette of the STATE of NEW SOUTH WALES Number 29 Friday, 6 February 2009 Published Under Authority by Government Advertising
559 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 29 Friday, 6 February 2009 Published under authority by Government Advertising LEGISLATION Announcement Online notification of the making of statutory instruments Following the commencement of the remaining provisions of the Interpretation Amendment Act 2006, the following statutory instruments are to be notified on the official NSW legislation website (www.legislation.nsw.gov.au) instead of being published in the Gazette: (a) all environmental planning instruments, on and from 26 January 2009, (b) all statutory instruments drafted by the Parliamentary Counsel’s Office and made by the Governor (mainly regulations and commencement proclamations) and court rules, on and from 2 March 2009. Instruments for notification on the website are to be sent via email to [email protected] or fax (02) 9232 4796 to the Parliamentary Counsel's Office. These instruments will be listed on the “Notification” page of the NSW legislation website and will be published as part of the permanent “As Made” collection on the website and also delivered to subscribers to the weekly email service. Principal statutory instruments also appear in the “In Force” collection where they are maintained in an up-to-date consolidated form. Notified instruments will also be listed in the Gazette for the week following notification. For further information about the new notification process contact the Parliamentary Counsel’s Office on (02) 9321 3333. 560 LEGISLATION 6 February 2009 Proclamations New South Wales Proclamation under the Brigalow and Nandewar Community Conservation Area Act 2005 MARIE BASHIR,, Governor I, Professor Marie Bashir AC, CVO, Governor of the State of New South Wales, with the advice of the Executive Council, and in pursuance of section 16 (1) of the Brigalow and Nandewar Community Conservation Area Act 2005, do, by this my Proclamation, amend that Act as set out in Schedule 1. -

Original Council Agenda
NOTICE OF ORDINARY MEETING Notice is hereby given that a meeting of Will be held at the Gloucester Administration Centre, 89 King Street, Gloucester 28 JUNE 2017 AT 2.00PM The order of the business will be as detailed below (subject to variation by Council) 1. Acknowledgement of Country 2. Declaration of Pecuniary or Conflicts of Interest (nature of Interest to be Disclosed) 3. Apologies 4. Confirmation of Minutes 5. Matters Arising from Minutes 6. Address from the Public Gallery 7. Matters for Information 8. Close of Meeting Glenn Handford INTERIM GENERAL MANAGER THIS PAGE IS LEFT BLANK INTENTIONALLY TABLE OF CONTENTS CONSIDERATION OF OFFICERS’ REPORTS: ....................................................................................... 2 DIRECTOR PLANNING & NATURAL SYSTEMS .................................................................................... 2 1 PLANNING PROPOSAL TO AMEND GREAT LAKES LEP & DCP - FORESHORE BUILDING LINE ........ 2 2 PLANNING PROPOSAL - HAWKS NEST VILLAGE AND OTHER ZONING OPPORTUNITIES............... 12 3 PLANNING PROPOSAL - CIVIC PRECINCT PROJECT, LAKE & WEST STS FORSTER ....................... 42 4 HOUSING DIVERSITY & AFFORDABILITY STRATEGY - COMMENCEMENT REPORT ........................ 44 DIRECTOR ENGINEERING & INFRASTRUCTURE .............................................................................. 48 5 CEDAR PARTY CREEK BRIDGE REPLACEMENT - PREFERRED DESIGN OPTION ........................... 48 6 CAPITAL WORKS REPORT - MAY, JUNE & JULY 2017 .........................................................................