How Does the Structure of Extraocular Muscles and Their Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Treatment of Congenital Ptosis
13 Review Article Page 1 of 13 Treatment of congenital ptosis Vladimir Kratky1,2^ 1Department of Ophthalmology, Queen’s University, Kingston, Canada; 21st Medical Faculty, Charles University, Prague, Czech Republic Correspondence to: Vladimir Kratky, BSc, MD, FRCSC, DABO. Associate Professor of Ophthalmology, Director of Ophthalmic Plastic and Orbital Surgery, Oculoplastics Fellowship Director, Queen’s University, Kingston, Canada; 1st Medical Faculty, Charles University, Prague, Czech Republic. Email: [email protected]. Abstract: Congenital ptosis is an abnormally low position of the upper eyelid, with respect to the visual axis in the primary gaze. It can be present at birth or manifest itself during the first year of life and can be bilateral or unilateral. Additionally, it may be an isolated finding or part of a constellation of signs of a specific syndrome or systemic associations. Depending on how much it interferes with the visual axis, it may be considered as a functional or a cosmetic condition. In childhood, functional ptosis can lead to deprivation amblyopia and astigmatism and needs to be treated. However, even mild ptosis with normal vision can lead to psychosocial problems and correction is also advised, albeit on a less urgent basis. Although, patching and glasses can be prescribed to treat the amblyopia, the mainstay of management is surgical. There are several types of surgical procedure available depending on the severity and etiology of the droopy eyelid. The first part of this paper will review the different categories of congenital ptosis, including more common associated syndromes. The latter part will briefly cover the different surgical approaches, with emphasis on how to choose the correct condition. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
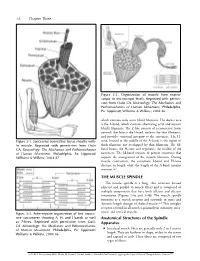
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Orbital Imaging in Thyroid-Related Orbitopathy
Orbital imaging in thyroid-related orbitopathy Christopher Lo, MD, Shoaib Ugradar, MD, and Daniel Rootman, MD, MS SUMMARY A broad understanding of the different imaging modalities used to assess the physiologic changes seen in Graves’ orbitopathy complement clinical examination. Subtle applications of radiographic imaging techniques allow for a better understanding of the overall physiology of the orbit, quantify progression of disease, and differentiate it from orbital diseases with overlapping features. A nuanced approach to interpreting imaging features may allow us to delineate inactive from active thyroid eye disease, and advances within this field may arm clinicians with the ability to better predict and prevent dysthyroid optic neuropathy. ( J AAPOS 2018;22:256.e1-256.e9) rbital imaging plays a central role in the diag- mean inferior rectus width, 4.8 mm; medial rectus width, nosis and management of thyroid-related orbit- 4.2 mm; superior rectus width, 4.6 mm; and lateral rectus O opathy (TRO). Diagnostically, it is used to width, 3.3 mm.8,9 These numbers can be used as a guide; compliment a careful ophthalmic examination, laboratory however, they represent population averages, each with values, and ancillary studies to confirm the presence of significant variation. Overlap in populations exist, and TRO and/or dysthyroid optic neuropathy (DON). It can both diseased and nondiseased muscles can have widths also be helpful in surgical planning and understanding close to these values. In the end, there are no strict rules. the progression of thyroid myopathy. Computed tomogra- In terms of muscle involvement, clinical myopathy is phy (CT), magnetic resonance imaging (MRI), ultrasound, thought to most often involve the inferior rectus muscle, and nuclear medicine all have applications in the field. -

Effect of Lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’
0270.6474/85/0507-1881$02.00/O The Journal of Neurowence CopyrIght 0 Society for Neuroscience Vol. 5, No. 7, pp. 1881-1885 Printed in U.S.A. July 1985 Effect of lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’ R. E. POPPELE*V2 AND D. c. QUICK* * Laboratory of Neurophysiology and $ Department of Anatomy, University of Minnesota, Minneapolis, Minnesota 55455 Abstract Materials and Methods Spindles were dissected free from tenuissimus muscles taken from anes- Sensitivity differences between primary and secondary thetized cats (pentobarbttal sodium, Nembutal, Abbott Laboratories, 35 mg/ endings of mammalian muscle spindles under various con- kg, or ketamine hydrochloride, Parke, Davis, 20 mg/kg). The isolated recep- ditions of stretch and fusimotor activation may be due to tor, together with about 1 cm of nerve, was mounted in a small chamber by differences in their respective mechanoelectric transducers tying each pole (near the capsule sleeve) to a small tungsten wire shaft or to mechanical properties of the intrafusal muscle support- connected to a servo-controlled Ling vibrator (model 108). The chamber was ing those endings. This study of isolated cat muscle spindles continuously perfused with oxygenated, modified Krebs’ solution (Poppele et al., 1979). The nerve was drawn onto a pair of electrodes in an adjacent examines the strain in individual intrafusal muscle fibers chamber containtng a high density fluorocarbon compound (FC-80, 3M Co.). resulting from stretch and fusimotor stimulation. The degree The entire assembly was mounted on a Zeiss photomicroscope equipped of local stretch occurring at the sensory endings under these with tine camera and Nomarski optics (see Poppele et al., 1979, and Poppele conditions was measured. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -

Extraocular Muscles. Conjugated Eye Movements. Strabismus
Extraocular muscles. Conjugated eye movements. Strabismus. Sándor Katz M.D., Ph.D. Conjugated eye movements Requirements: • Extraocular muscles • Innervation: CN. III., IV., VI. Precision and speed of muscle function rely on structural characteristics. Their motor innervation is well developed: 1 motor fiber/6 muscle fibers (in finger: 100-300 muscle fibers, in other muscles: 1500 muscle fibers) • Central pathways for eye movements, ensuring coordination Orbit Eye vs. Orbit • The medial wall runs in paramedian sagittal plane. • The lateral wall deviates laterally: 45°. • The AP (antero- posterior) axis of the eye is parallel with the sagittal axis. • The extraocular muscles run almost parallel with the axis of orbit. Orbit Orbit The eyeball is attached by membranes to the capsule of the orbital fat body, and it can move in all directions in the episcleral space. Movements are achieved by four recti and two oblique muscles. The tendons of the rectus muscles originate form a funnel-shaped ring around the optic canal: common tendinous ring. Orbit medial rectus nasociliary nerve superior rectus superior oblique lateral rectus lacrimal nerve frontal nerve lacrimal gland superior oblique medial rectus inferior rectus inferior oblique pes anserinus minor Possible eye movements Sagittal axis: Internal/medial rotation External/lateral rotation (difficult to see as the pupil is round in humans). Possible eye movements Horisontal axis: Elevation Depression Possible eye movements Vertical axis: Abduction Adduction Lateral rectus muscle – CN VI. Vertical axis: Abduction Origin: common tendinous ring Insertion: sclera, posterior to the limbus corneae Medial rectus muscle – CN III., inferior ramus Vertical axis: Adduction Origin: common tendinous ring Insertion: sclera, posterior to the limbus corneae Superior rectus muscle – CN. -

Double-Bellied Superior Rectus Muscle
Surgical and Radiologic Anatomy (2019) 41:713–715 https://doi.org/10.1007/s00276-019-02211-0 ANATOMIC VARIATIONS Double-bellied superior rectus muscle Satheesha B. Nayak1 · Surekha D. Shetty1 · Naveen Kumar1 · Ashwini P. Aithal1 Received: 3 September 2018 / Accepted: 23 February 2019 / Published online: 7 March 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Congenital variations of extraocular muscles are rare. We report a double-bellied superior rectus muscle, observed in an adult male cadaver aged 70 years. The superior rectus muscle had two equal-sized bellies, which took separate origins from the common tendinous ring and united to form a common belly 1 cm before the insertion. Due to the duplication, the muscle extended laterally beyond the levator palpebrae superioris. Both its bellies were supplied by oculomotor nerve. To the best of our knowledge, this is the first report on doubling of the belly of the superior rectus muscle. Keywords Extraocular · Orbit · Superior rectus muscle · Eye movement · Strabismus Introduction Case report Voluntary movements of the eyeball are performed by six During dissection classes for the first-year medical students, extraocular muscles, namely superior rectus muscle, the we observed a unique variation in the right orbit of an adult inferior rectus muscle, medial rectus muscle, lateral rectus male cadaver aged 70 years. The cadaver was donated to the muscle, superior oblique muscle, and inferior oblique mus- department for teaching and research purpose. No history of cles. Variations of these muscles can result in restrictions of strabismus or visual defects is available. The variation was movements of eyeball, causing strabismus. -

Related Disease of the Orbit: Imaging Features in 27 Patients
Published March 13, 2014 as 10.3174/ajnr.A3865 ORIGINAL RESEARCH HEAD & NECK Immunoglobulin G4–Related Disease of the Orbit: Imaging Features in 27 Patients C.A. Tiegs-Heiden, L.J. Eckel, C.H. Hunt, F.E. Diehn, K.M. Schwartz, D.F. Kallmes, D.R. Saloma˜o, T.E. Witzig, and J.A. Garrity ABSTRACT BACKGROUND AND PURPOSE: Immunoglobulin G4–related disease is a systemic fibroinflammatory process of unknown etiology, characterized by tissue infiltration by immunoglobulin G4 plasma cells. The purpose of this study was to retrospectively identify the spectrum of imaging features seen in immunoglobulin G4–related disease of the orbit. MATERIALS AND METHODS: This study included 27 patients with biopsy-proved immunoglobulin G4–related disease of the orbit and either a CT or MR imaging of the orbits. These CT or MR imaging examinations were evaluated for the following: extraocular muscle size, extraocular muscle tendon enlargement, lacrimal gland enlargement, infiltrative process in the orbital fat (increased attenuation on CT or abnormal signal on MR imaging), infraorbital nerve enlargement, mucosal thickening in the paranasal sinuses, and extension of orbital findings intracranially. RESULTS: Extraocular muscles were enlarged in 24 of 27 (89%) patients, 21 (88%) bilaterally. In 32 of 45 (71%) affected orbits, the lateral rectus was the most enlarged muscle. In 26 (96%) patients, the tendons of the extraocular muscles were spared. Nineteen (70%) patients had lacrimal gland enlargement. Twelve (44%) patients had an infiltrative process within the orbital fat. Infraorbital nerve enlargement was seen in 8 (30%) patients. Twenty-four (89%) patients had sinus disease. Cavernous sinus or Meckel cave extension was seen in 3 (11%) patients.