Supplementary Materials Hierarchical Nanocauliflower Chemical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Day Amrit Campus 2075
62 nd Annual Day Sovenir 2075 ASCOLITE Amrit Campus IOST, Tribhuvan University Dedicated to Prof. Amrit Prasad Pradhan ( 1918, Nov, 3 Sunday to 24th, January 1966) hGd ldtL lj=;+= !(&% sftL{s !* ut] cfOtjf/ :j= lj=;+= @)@@ df3 !* ut] ;f]djf/ Content S.N. Particular Name Page 1 Amrit Campus from editorial desk 2 Contemporary Journey of Amrit Campus and Necessity for Dr. Puspa Lal Homagai 1 10 Revampment 3 A brief history of amrit campus with recent education activities. Rabindra Singh Mahat 11 4 Past and Present Status of Physics Department Asst Prof. Dr. Leela Pradhan 13 5 Present Status of Department of Chemistry Amrit Campus, TU Kiran Bahadur Bajracharya 15 Kathmandu 6 Department of Botany, Prof. Dr.Mukesh K Chettri and 19 Prof. Dr Kanta Poudyal 7 Department of Zoology at Amrit Campus Prof. Dr. Shyam Narayan Labh 24 8 Environmental Science at Amrit Campus, Tribhuvan University 29 9 Microbiology 31 10 Mathematics 32 11 Department of Computer Science and Information Technology Mr. Binod Kumar Adhikari 33 Amrit Campus, Tribhuvan University 12 Amrit Hostel at a Glance Umakant Lal Karna 34 13 Campus Library at a Glance Ramesh Niraula 35 14 lq=lj= k|fWofks ;+3 cd[t SofDk; PsfO ;ldlt Ps emns kjg s'df/ emf 39 15 lq=lj= sd{rf/L ;+3 cd[t SofDk; PsfO{ ;ldltsf] e"ldsf s'df/ >]i7 40 16 sNof0fsf/L / lzIffk|b v]ns'b ljlkg s'df/ bfxfn 41 17 Administration Staff 43 18 Teaching Staff 44 TRIBHUVAN UNIVERSITY Kirtipur, Kathmandu, Nepal OFFICE OF THE VICE CHANCELLOR August 14th, 2018 Message from the Vice Chancellor I am rejoiced to know that Amrit Campus,under Institute of Science and Technologyis publishing this issue of 62th Annual Day. -

Click Here to Download
Tribhuvan University Kirtipur, Kathmandu, Nepal July 17, 2013 Message from the Vice-Chancellor It gives me immense pleasure that TU Today is coming up with the updated information on Tribhuvan University (TU) in its 54th year of establishment. On this occasion, I would like to thank the Information Section, TU, and all those involved in the publica- tion of TU Today. Furthermore, I take this opportunity to express my gratitude to the teaching faculty whose relentless work, dedication and honest contribution has helped the university open up innovative academic programmes, maintain the quality of education, and enhance teaching and research. I also thank the administrative staff for effi ciently bearing the management responsibility. Nonetheless, I urge the faculty and the staff for their additional devotion, commitment and effi ciency to retain TU as one of the quality higher education institutions in the country. It is an objective reality among us that TU has been the fi rst choice of a large number of students and guardians for higher education. I sincerely thank for their trust on TU for higher education and express my unwavering determination and commitment to serve them the best by providing excellent academic opportunity. I would like to urge the students to help the university maintain its academic ethos by managing politics, maximiz- ing learning activities, and respecting the ideals of university without condition. Despite its commitment to enhance and impart quality education, TU faces many challenges in governance and resource management for providing basic infrastructure and educational facilities required for quality education environment. In spite of limited infrastructures and educational facilities, it has been producing effi cient and competent graduates. -

Quality Assurance and Accreditation in Higher Education of Nepal: Practices and Issues
Quality Assurance and Accreditation in Higher Education of Nepal: Practices and Issues Pradhan, Bijay Lal 1*, Malla Pradhan, Rajeshwori2. 1 Associate Professor, Tribhuvan University, Amrit Campus, Thamel, Kathmandu, Nepal 1 General Secretary, NQPCN, 2 Tri Chandra Campus, Ghantaghar, Kathmandu, Bagmati Province, Nepal *Corresponding Author: [email protected] Abstract The first higher educational institute in Nepal is Tri Chandra Campus, which was established in the year 1918 and which was accessible to a handful people till the establishment of Tribhuvan University in the year 1959. Regarding The Higher Educational Institute (HEI) in Nepal has diversified by different aspects. Universities of Nepal is offering different program as well the Institutions are awarding certificate in the specific field of health science. Similarly, there is diversity in the colleges also as constituent colleges, Community colleges and private college. All together there are 11 universities, 4 award giving institution and 1407 colleges exist throughout the nation. University Grant Commission Nepal (UGC-Nepal) was established after the Multi University Concept was introduced in Nepal. Regarding the function of UGC, it is responsible for allocation and disbursement of grants to the universities and their campuses. In the same way it regulates the activities of universities. i.e. maintenance of standard of teaching, examination and research activities in universities. Similarly, it is working on formulating policies and program for universities of Nepal. Regarding the governing the Higher education in the country there are different concerns; they are: regulations, governance, excellence, funding and quality. This paper will focus on quality assurance aspect for governing the HEIs of Nepal. The University Grants Commission (UGC), Nepal has launched the Quality Assurance and Accreditation (QAA) programme, as an important aspect of reform in higher education in Nepal. -

International Conference on Invasive Alien Species Management
Proceedings of the International Conference on Invasive Alien Species Management NNationalational TTrustrust fforor NatureNature ConservationConservation BBiodiversityiodiversity CConservationonservation CentreCentre SSauraha,auraha, CChitwan,hitwan, NNepalepal MMarcharch 2525 – 227,7, 22014014 Supported by: Dr. Ganesh Raj Joshi, the Secretary of Ministry of Forests and Soil Conserva on, inaugura ng the conference Dignitaries of the inaugural session on the dais Proceedings of the International Conference on Invasive Alien Species Management National Trust for Nature Conservation Biodiversity Conservation Centre Sauraha, Chitwan, Nepal March 25 – 27, 2014 Supported by: © NTNC 2014 All rights reserved Any reproduc on in full or in part must men on the tle and credit NTNC and the author Published by : NaƟ onal Trust for Nature ConservaƟ on (NTNC) Address : Khumaltar, Lalitpur, Nepal PO Box 3712, Kathmandu, Nepal Tel : +977-1-5526571, 5526573 Fax : +977-1-5526570 E-mail : [email protected] URL : www.ntnc.org.np Edited by: Mr. Ganga Jang Thapa Dr. Naresh Subedi Dr. Manish Raj Pandey Mr. Nawa Raj Chapagain Mr. Shyam Kumar Thapa Mr. Arun Rana PublicaƟ on services: Mr. Numraj Khanal Photo credits: Dr. Naresh Subedi Mr. Shyam Kumar Thapa Mr. Numraj Khanal CitaƟ on: Thapa, G. J., Subedi, N., Pandey, M. R., Thapa, S. K., Chapagain, N. R. and Rana A. (eds.) (2014), Proceedings of the InternaƟ onal Conference on Invasive Alien Species Management. Na onal Trust for Nature Conserva on, Nepal. This publica on is also available at www.ntnc.org.np/iciasm/publica ons ISBN: 978-9937-8522-1-0 Disclaimer: This proceeding is made possible by the generous support of the Asian Development Bank (ADB), the American people through the United States Agency for InternaƟ onal Development (USAID) and the NaƟ onal Trust for Nature ConservaƟ on (NTNC). -

279 Members, As of July 2021
List of IPSI members 279 members (as of July 2021) Organisation Location of head office National governmental organisation (Number of organisations 22) Environment Protection Authority, Yemen Yemen Ethiopian Biodiversity Institute Ethiopia Ghana National Biodiversity Committee Ghana Ministry of Agriculture, Food and Forestry Policies, Italy Italy Ministry of Commerce, Industry and Environment, Directorate General for Environment, Timor Leste Timor-Leste Ministry of Environment and Forest Resources, Togo Togo Ministry of Environment and Protection of Nature, Cameroon Cameroon Ministry of Environment and Tourism, Mongolia Mongolia Ministry of Environment and Water Resources, Chad Chad Ministry of Environment, Cambodia Cambodia Ministry of Environment, Ecology and Forests, Madagascar Madagascar Ministry of Environment, Gabon Gabon Ministry of Environment, Peru Peru Ministry of Environment, Republic of Korea Republic of Korea Ministry of Forestry and Environment, Gambia Gambia Ministry of Forests and Soil Conservation, Nepal Nepal Ministry of Natural and Resources and Environment, Thailand Thailand Ministry of Natural Resources, Energy and Mining, Malawi Malawi Ministry of the Environment, Japan Japan National Environmental Council for Sustainable Development, Executive Secretariat, Niger Niger National Forest and Wildlife Service (SERFOR), Peru Peru National System of Conservation Areas (SINAC), Costa Rica Costa Rica Other government affiliated organisation (Number of organisations 9) Fisheries Research Institute, Council of Agriculture, -

March 13Th -14Th, 2021 About SONSIK
March 13th -14th, 2021 About SONSIK Since 1990 Nepalese students had started studying in South Korea. However, after 2000 only the students flow at South Korea was increased rapidly. Even after rapid increase of students flow at South Korea there did very few students know each other and less opportunity to share knowledge/ experience gained after coming in Korea. On 2004 group of intellectuals from different university gathered at Sun Moon University, Cheonan Korea, after deep thought and discussion Society of Nepalese Students in Korea (SONSIK) was established and had its first official meeting at Sun Moon University. Initially the goal of SONSIK was to have frequent meeting with different Nepalese scholars in Korea with the changing time the mission which began 17 years ago is still the goal today to share/strengthen the bond and knowledge between more than 5000 fellow members of Korean Universities. Furthermore, the goal is set a step ahead to make SONSIK the only intellectual organization where the policy maker can look up to. Over the past years we have grown beyond Korean peninsula and our effort have not gone unnoticed. For the proper functioning of the organization SONSIK has an annual basis formal structural executive body to manage indented plans. Please click http://sonsik.org.np/ for detail about our organization. SONSIK 8th Educational Seminar 2021 1 Virtual Conference About Educational Seminar The Society of Nepalese Students in Korea (SONSIK), being the sole community of the Nepalese students and academicians in Korea, is working continuously for the promotion of Nepalese students studying in South Korea with different academic, leadership development, social networking, educational seminars, and refreshment programs. -

Proceedings of the First National Conference on Zoology
1 Biodiversity in a Changing World Proceedings of First National Conference on Zoology 28-30 November 2020 Published By Central Department of Zoology Institute of Science and Technology, Tribhuvan University Kathmandu, Nepal Supported By “Biodiversity in a Changing World” Proceedings of the First National Conference on Zoology 28–30 November 2020 ISBN: Published in 2021 © CDZ, TU Editors Laxman Khanal, PhD Bishnu Prasad Bhattarai, PhD Indra Prasad Subedi Jagan Nath Adhikari Published By Central Department of Zoology Institute of Science and Technology, Tribhuvan University Kathmandu, Nepal Webpage: www.cdztu.edu.np 3 Preface The Central Department of Zoology, Tribhuvan University is delighted to publish a proceeding of the First National Conference on Zoology: Biodiversity in a Changing World. The conference was organized on the occasional of the 55 Anniversary of the Department from November 28–30, 2020 on a virtual platform by the Central Department of Zoology and its Alumni and was supported by the IUCN Nepal, National Trust for Nature Conservation, WWF Nepal and Zoological Society of London Nepal office. Faunal biodiversity is facing several threats of natural and human origin. These threats have brought widespread changes in species, ecosystem process, landscapes, and adversely affecting human health, agriculture and food security and energy security. These exists large knowledge base on fauna of Nepal. Initially, foreign scientist and researchers began explored faunal biodiversity of Nepal and thus significantly contributed knowledge base. But over the decades, many Nepali scientists and students have heavily researched on the faunal resources of Nepal. Collaboration and interaction between foreign researchers and Nepali researchers and students are important step for further research and conservation of Nepali fauna. -

Curriculum-Vitae Krishna Prasad Bhandari Date of Birth: 04-08-1968
Curriculum-Vitae Krishna Prasad Bhandari Date of Birth: 04-08-1968 Place of Birth: Dhikurpokhari-4 Kaski,Nepal Nationality: Nepalese Address Correspondence Western Region campus Lamachour, Pokhara -16, Nepal E-mail [email protected] Permanent Dhikurpokhari-4 kaski, Nepal Academic Qualifications Degree Subjects From To Institute Address Environmental Faculty of Environmental Management/PGI June, July, 90112, HatYai, PhD Management, Prince of S for soil erosion 2010 2014 Shongkla,Thailand Songkla University managent Centre for Space science Post Graduate 1, and Technology 4 kalidas Road, Remote Sensing 30, June Diploma in RS October Education in Asia Pacific Dehradun, and GIS 2007 and GIS 2006 (CSSTEAP UN Uttrakhand,India Affiliated), IIRS. Master’s Central Department of Degree in Kirtipur, Mathematics 1992 1994 Mathematics, Tribhuvan Science (M. Kathmandu University, Nepal Sc. ) Bachelor’s Physics,Chem,Ma Tribhuvan University Lainchaur, degree in 1989 1991 ths Amrit Campus ,Nepal Kathmandu Science ( B. Sc. ) Bachelor’s Tribhuvan University, Degree in Mathematics 1996 1997 Prithvi Narayan Multiple Pokhara-1 Kaski Education (B. Campus, Nepal Ed.) Experience • Associate Professor at Institute of Engineering, Western Region Campus Lamachaur, Pokhara, Nepal since 2012-04-19. • Teaching Assistance in Faculty of Environmental Management, from June 2010 to October 2010, Prince of Songkla University, Hatyai, Thailand. • Lecturer in Mathematics at the Institute of Engineering, Western Region Campus Lamachaur, Pokhara, Nepal since 1996-09-07 to 2012-04-18. • Head of the Department Science and Humanities at the Institute of Engineering, Western Region Campus, Lamachaur, Pokhara, Nepal since 2007 -11-15 to 2010-03- • Visiting faculty at summer winter school of CEPT University, India "Rethinking Pokhara after Earthquake" course at Faculty of Technology from 1 Dec, 2015 to 13 Dec, 2015. -

Technological Characterisation of Roofing Slate from Nepal
Journal of Nepal Geological Society, 2016, Vol. 50, pp. 19-23 Technological characterisation of roofing slate from Nepal Technological characterisation of roofing slate from Nepal *Victor Cardenes1, Naba Raj Neupane², Lalu P. Paudel³, Veerle Cnudde1, Jean Pierre Cnudde1 1UGCT/PproGress, Department of Geology and Soil Science, Ghent University, Krijgslaan 281/S8, 9000 Ghent, Belgium ²Department of Environmental Science, Amrit Campus, Tribhuvan University, Kathmandu, Nepal ³Central Department of Geology, Tribhuvan University, Kathmandu, Nepal (Email: [email protected]) ABSTRACT Roofing slate is a group of natural stones which can be split into thin, large and regular tiles. The world’s slate market is the third in importance for natural stone, just after granite and marble. Most of the world’s production of roofing slate is concentrated in Spain. There are new emerging countries (Brazil, China, India and Nepal) with huge resources of roofing slate. However, many of these resources are not yet well studied. Nepalese slate industry is still incipient, although most of the houses have been constructed using roofing slate since historic time. The representative three samples were taken for quality assessment from Tanahun and Baglung districts. From a geological point of view, the productive formations are the Benighat Slates and Nourpul Formation, but other formations are likely to be potential slate sources. Previous studies have shown an important reserve of slate which needs detailed investigation. Petrography and mineralogy of representative Nepalese slates are similar to the rest of the world’s roofing slate. The test results showed values within the acceptance thresholds for roofing slates. This paper also compares the Nepalese slates with other varieties of roofing slates from Spain. -

Wavelet and Cross Correlation Analysis on Some Climatology Parameters of Nepal Babu Ram Tiwari1*, Jiyao Xu1, Binod Adhikari2, Narayan P
BIBECHANA 18 (2) (2021)105-116 Wavelet and cross correlation analysis on some climatology parameters of Nepal Babu Ram Tiwari1*, Jiyao Xu1, Binod Adhikari2, Narayan P. Chapagain3 1State Key Laboratory of Space Weather, National Space Science Center, Chinese Academy of Sciences, Beijing, China- 100190 2Department of Physics, St. Xavier’s College, Maitighar , Kathmandu, Nepal 3Department of Physics, Amrit Campus, Tribhuvan University, Kathmandu, Nepal Email: [email protected] Article Information: ABSTRACT Received:December 27, 2020 This study has been performed to understand the relationship between sunspot Accepted: May 28, 2021 numbers (SSN) with climatology related parameters like temperature and rainfall from 1901 to 2016. The spectral characteristics of sunspot numbers, temperature Keywords: and rainfall have been observed using continuous wavelet transform. Cross- Climate variability correlation analyses were also performed to find any relation among temperature, Wavelet transform rainfall, and sunspot numbers. The 9–11 year periodicity of sunspot numbers Cross-correlation analysis confirmed by wavelet transform in annual scale. The periodicity of high- frequency signals is identified between 4 to 11 years whereas the low frequencies signal is found throughout the periods of observation for temperature. Similarly, it is clear that there is more concentration of power between 8–16 years for rainfall. Cross-correlation analysis shows that the sunspot numbers is highly correlated with rainfall and temperature (correlation coefficient ~ 0.8054). The time lag relationship resulted in the almost simultaneous linear relationship between the temperature, rainfall, and the SSN tendency. Development of convective motions over the subtropics might be affected by the time rate of change of SSN combined with the surface temperature changes of diverse time scales. -
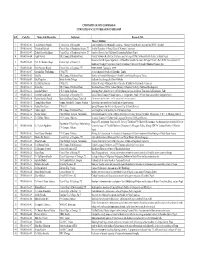
Research Recodr
UNIVERSITY GRANTS COMMISSION COMPLETED FACULTY RESEARCH UNDER SHEP S.N Code No. Name of the Researcher Institution Research Title Phase I (065/066) 1 FR-065/066-01 Umesh Kumar Mandal Central Dept. of Geography Land Evaluation For Sustainable Land use Planning Using Remote Sensing And GIS Technology 2 FR-065/066-02 Trilochan Pokharel Central Dept. of Population Studies, TU Fertility Transition In Nepal: Role Of Women’s Autonomy 3 FR-065/066-03 Keshab Prasad Adhikari Central Dept. of Population Studies, TU Gendered Poverty And Childhood Deprivation In Rural Nepal. 4 FR-065/066-04 Kapil Gyawali R.R. Campus, Exhibition Road Current Newborn Health Care Practices And Coverage Of The Newborn Health Service In Rural Nepal. Historical And Religious Significance Of Buddhist Sanskrit Literature Of Nepal: Nepal’s Role In The Development Of 5 FR-065/066-05 Prof. Dr. Shankar Thapa Central Dept. of History, TU Buddhism Through Preservation And Proliferation Of Literary Of Sanskrit Buddhist Manuscripts. 6 FR-065/066-06 Bhim Narayan Regmi Central Dept. of Linguistic, TU g]Kffnsf Effiffx?sf] Typological cWoog 7 FR-065/066-07 Govinda Bdr. Tumbahang CNAS, TU A Sociolinguistic Study of Chhatthare Limbu 8 FR-065/066-08 Bina Rai R.R. Campus, Exhibition Road Male Involvement In Reproductive Health Under Uterus Prolapsed Threat. 9 FR-065/066-09 Gita Thapaliya British Gorkha College Aadhunik Geet Sangeet ko Manak Pakchya 10 FR-065/066-10 Dr. Dilli Raj Sharma CNAS, TU Cultural Heritage Of Bagmati River Corridor: A Study On Tourism Development 11 FR-065/066-11 Shanta Rai R.R. -

Himalayan Journal of Sciences
Volume 2 Issue 3 Jan-June 2004 ISSN 1727 5210 editorial correspondence Let’s air our dirty laundry Chemical research should be a Scientists and developers can’t save the world national priority when they have to play along to get along Rajendra Uprety Seth Sicroff Page 10 Page 9 essay policy Himalayan Journal of Sciences Volume 2, Issue 3 Scientists: Four golden lessons Theories for sustainable futures Jan-June 2004 Advice to students at the start of their Sustainable development requires integration of Pages: 1-70 scientific careers ecological, economic and social theories Steven Weinberg C S Holling Cover image credit: Page 11 Page 12 Krishna K Shrestha resource review special announcement How to control illegal wildlife trade in the Mountain Legacy announces plans Himalayas Mountain Legacy announces plans for conference As Nepal’s greatest natural resources approach on Mountain Hazards and Mountain Tourism, extinction, the stakes could hardly be higher calls for nominations for second Hillary Medal, Ram P Chaudhary and proposes research and development institute Page 15 in Rolwaling Page 20 publication preview Published by Himalayan perceptions: Environmental change and the well-being of mountain peoples Himalayan Association for Fifteen years ago, the Himalayan Dilemma buried the most popular environmental paradigm of the 80s. the Advancement of Science What will it take for policy-makers to get the message? Lalitpur, Nepal Jack D Ives GPO Box No. 2838 Page 17 HIMALAYAN JOURNAL OF SCIENCES VOL 2 ISSUE 3 JAN-JUNE 2004 7 Seth Sicroff