Antioxidant, Anti-Inflammatory Activities and HPLC Quantification of Flavonoids in Pteris Tripartita Sw
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Vitro Spore Germination and Gametophytic Growth Development of a Critically Endangered Fern Pteris Tripartita Sw
Vol. 13(23), pp. 2350-2358, 4 June, 2014 DOI: 10.5897/AJB2013.13419 Article Number: 6C227C945161 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Full Length Research Paper In vitro spore germination and gametophytic growth development of a critically endangered fern Pteris tripartita Sw. Baskaran Xavier Ravi*, Jeyachandran Robert and Melghias Gabriel Department of Botany, St. Joseph’s College, Tiruchirappalli, Tamil Nadu-620 002, India. Received 24 October, 2013; Accepted 31 March, 2014 The effects of sucrose, pH and plant growth hormones on spore germination percentage and gametophyte growths of Pteris tripartita were studied. Various morphological structures of gametophytes were observed namely, filamentous, spatulate and heart stages in the MS culture medium with hormones. After 15 days, the spores of P. tripartita were sprouted in MS basal medium fortified with pH, sucrose and hormones. Maximum spore germination rates (84%) were observed in 70 g/L of sucrose and 79.33% in pH 5.7. On the other hand, the maximum gametophyte sizes were observed both in 40 and 50 g/l of sucrose on half strength MS medium. The maximum growth of gametophyte lengths (484.39 and 507.72 µm) and widths (846.58 and 1270.98 µm) were observed in both pH 5.7 and 6.7. Among three different hormones, the utmost number or percentage of spores were sprouted in GA3. However, the in vitro cultures of spore having the capability to increase the spore germinated due to addition of adequate nutrition in the culture medium and also reduce the contamination as well as environmental factors. -

Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia
THE JOURNAL OF TROPICAL LIFE SCIENCE OPEN ACCESS Freely available online VOL. 5, NO. 2, pp. 98-104, May, 2015 Gametophyte Morphology and Development of Six Species of Pteris (Pteridaceae) from Java Island Indonesia Dwi Sunarti Puspitasari1, Tatik Chikmawati2*, Titien Ngatinem Praptosuwiryo3 1Plant Biology Graduate Program, Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 2Department of Biology, Faculty of Mathematics and Natural Sciences Bogor Agricultural University, Darmaga Campus, Bogor, Indonesia 3Center for Plant Conservation- Bogor Botanical Gardens, Indonesian Institute of Sciences, Bogor, West Java, Indonesia ABSTRACT The morphology of sporophyte, the type of reproduction, and cytology of Pteris had been reported, while the gametophyte morphology of Pteris in Java island has not been studied yet. The objective of this study was to describe the gametophyte morphology and development of P. biaurita, P. ensiformis, P. exelsa, P. longipinnula, P. tripartita, and P. vittata in Java island. Spores were obtained from fertile leaves of Pteris plants originated from several locations in Java island. The number of spores per sporangium was counted from fresh fertile leaves with mature sporangia. As much as 0.002 g spores was sown in a transparent box with sterile medium contain of ver- miculite, sphagnum moss, and perlite with ratio 2:2:1. The gametophyte development of each species was observed under a microscope every 7 days. The spores of P. ensiformis were germinated faster, ten days after sowing, while the spores of P. longipinnula were germinated slower, 18 days after sowing. The pattern of spore germination is Vittaria-type. -
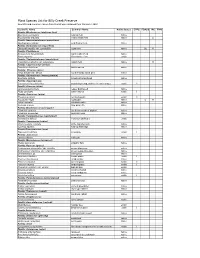
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

Characterization of Antimicrobial Compounds from a Common Fern, Pteris Biaurita
Indian Journal of Experimental Biology Vol. 45, March 2007, pp. 285-290 Characterization of antimicrobial compounds from a common fern, Pteris biaurita A K Dalli, G Saha & U Chakraborty* Plant Biochemistry Laboratory, Department of Botany, University of North Bengal, Siliguri 734013, India Received 29 May 2006; revised 5 December 2006 Methanol extract was prepared from the fronds of Pteris biaurita and partial purification was done by solvent partitioning with diethyl ether and ethyl acetate, followed by hydrolysis and further partitioning with ethyl acetate. The three fractions, thus obtained were bioassayed separately against five test fungi- Curvularia lunata, Fomes lamaoensis, Poria hypobrumea, Fuasrium oxysporum and a bacterium- Bacillus pumilus, by spore germination, radial growth and agar cup techniques. Results revealed that ethyl acetate fraction (III) contained the active principle. TLC plate bioassay of the active fraction revealed inhibition zone at an Rf of 0.5-0.65. Silica gel from this region was scraped, eluted in methanol and subjected to UV-spectrophotometric analysis. An absorption maxima of 278 nm was recorded. HPLC analysis of TLC- eluate revealed a single peak with retention time of 8.1 min. GC-MS analysis revealed six major peaks in the retention time range of 7.2-10.9 min. Comparison with GC-MS libraries revealed that the extracts may contain a mixture of eicosenes and heptadecanes. Keywords: Antimicrobial compounds, Fern, HPLC, GC-MS Pteris biaurita L., is a common fern that grows Bengal. Four of the five fungi were selected for luxuriantly under varying habitats and is used for bioassay as these are common plant pathogenic fungi, ornamental purposes. -

The Pteridaceae Family Diversity in Togo
Biodiversity Data Journal 3: e5078 doi: 10.3897/BDJ.3.e5078 Taxonomic Paper The Pteridaceae family diversity in Togo Komla Elikplim Abotsi‡, Aboudou R. Radji‡, Germinal Rouhan§, Jean-Yves Dubuisson§, Kouami Kokou‡ ‡ Université de Lomé, Lomé, Togo § Museum National d'Histoire Naturelle, Paris cedex 05, France Corresponding author: Komla Elikplim Abotsi ([email protected]) Academic editor: Daniele Cicuzza Received: 10 Apr 2015 | Accepted: 10 Jul 2015 | Published: 15 Jul 2015 Citation: Abotsi K, Radji A, Rouhan G, Dubuisson J, Kokou K (2015) The Pteridaceae family diversity in Togo. Biodiversity Data Journal 3: e5078. doi: 10.3897/BDJ.3.e5078 Abstract Background The Pteridaceae family is the largest fern family in Togo by its specific and generic diversity. Like all other families of ferns in the country, Pteridaceae are poorly studied and has no identification key. The objective of this study is to perform a taxonomic revision and list establishment of this family of leptosporangiate ferns in the light of current available knowledge about the family. Pteridaceae was also assessed in terms of its diversity and conservation status, this was conducted through the recent field data and the existing herbaria specimens. The current study permits to confirm the presence of Pteris similis Kuhn. which brought the number of Pteridaceae to 17 in Togo. New information This study provides first local scientific information about the fern flora of Togo. It confirmed the presence of Pteris similis Kuhn. in Togo and brought the Pteridaceae family diversity to 17 species. A species identification key is provided for the easy identification of the Pteridaceae of Togo. -

Ecology, Diversity and Taxonomy of the Pteridophytes of Pharenda Forest of Maharajganj District, Uttar Pradesh
International Journal of Research Studies in Biosciences (IJRSB) Volume 4, Issue 2, February 2016, PP 40-47 ISSN 2349-0357 (Print) & ISSN 2349-0365 (Online) http://dx.doi.org/10.20431/2349-0365.0402006 www.arcjournals.org Ecology, Diversity and Taxonomy of the Pteridophytes of Pharenda Forest of Maharajganj District, Uttar Pradesh Ravi Pratap Gautam1, S. Dominic Rajkumar2, Shobhit Kumar Srivastava3, Shashank Kumar Singh4, Akhilesh Kumar Gupta5 Department of Botany St. Andrew‟s College, Gorakhpur, Uttar Pradesh [email protected] Abstract: Eastern Uttar Pradesh is rich in plant diversity as most of its parts are situated at the foothills of Great Himalaya. The forests of these places are supposed to have abundant diversity of all types of plants from lower forms to higher. Pharenda Forest is one of them. It is a township also known as Anand Nagar situated in Maharajganj district of Uttar Pradesh. Geographically Pharenda is situated between the coordinates 27º 06’ N and 83º 17’ E. A survey consisting repetitive field trips was made to learn about the diversity and richness of Pteridophytes of this forest on the basis of effects of climate change. During this study about eleven species of Pteridophytes were collected and morphological observations were made. Keywords: Eastern Uttar Pradesh, plant diversity, Pharenda, Pteridophytes, climate change. 1. INTRODUCTION The Pteridophytes are one of the primitive vascular plants distributed all over the world. India consists of a plenty and diverse pteridophytic flora because of its various climatic conditions and geography. The ferns are found rich in the Himalayas and Western Ghats, as these places are the two hotspots of biodiversity in India. -

Biogeographical Patterns of Species Richness, Range Size And
Biogeographical patterns of species richness, range size and phylogenetic diversity of ferns along elevational-latitudinal gradients in the tropics and its transition zone Kumulative Dissertation zur Erlangung als Doktorgrades der Naturwissenschaften (Dr.rer.nat.) dem Fachbereich Geographie der Philipps-Universität Marburg vorgelegt von Adriana Carolina Hernández Rojas aus Xalapa, Veracruz, Mexiko Marburg/Lahn, September 2020 Vom Fachbereich Geographie der Philipps-Universität Marburg als Dissertation am 10.09.2020 angenommen. Erstgutachter: Prof. Dr. Georg Miehe (Marburg) Zweitgutachterin: Prof. Dr. Maaike Bader (Marburg) Tag der mündlichen Prüfung: 27.10.2020 “An overwhelming body of evidence supports the conclusion that every organism alive today and all those who have ever lived are members of a shared heritage that extends back to the origin of life 3.8 billion years ago”. This sentence is an invitation to reflect about our non- independence as a living beins. We are part of something bigger! "Eine überwältigende Anzahl von Beweisen stützt die Schlussfolgerung, dass jeder heute lebende Organismus und alle, die jemals gelebt haben, Mitglieder eines gemeinsamen Erbes sind, das bis zum Ursprung des Lebens vor 3,8 Milliarden Jahren zurückreicht." Dieser Satz ist eine Einladung, über unsere Nichtunabhängigkeit als Lebende Wesen zu reflektieren. Wir sind Teil von etwas Größerem! PREFACE All doors were opened to start this travel, beginning for the many magical pristine forest of Ecuador, Sierra de Juárez Oaxaca and los Tuxtlas in Veracruz, some of the most biodiverse zones in the planet, were I had the honor to put my feet, contemplate their beauty and perfection and work in their mystical forest. It was a dream into reality! The collaboration with the German counterpart started at the beginning of my academic career and I never imagine that this will be continued to bring this research that summarizes the efforts of many researchers that worked hardly in the overwhelming and incredible biodiverse tropics. -

Pteridophytic Diversity in Human-Inhabited Buffer Zone of Murlen National Park, Mizoram, India
13 2 2081 the journal of biodiversity data 3 April 2017 Check List LISTS OF SPECIES Check List 13(2): 2081, 3 April 2017 doi: https://doi.org/10.15560/13.2.2081 ISSN 1809-127X © 2017 Check List and Authors Pteridophytic diversity in human-inhabited buffer zone of Murlen National Park, Mizoram, India Sachin Sharma1, Bhupendra S. Kholia1, 4, Ramesh Kumar2 & Amit Kumar3 1 Botanical Survey of India, Northern Regional Centre, Dehradun 248 195, Uttarakhand, India 2 Botanical Survey of India, Arid Zone Regional Centre, Jodhpur 342 008, Rajasthan, India 3 Wildlife Institute of India, P.O. Box #18, Chandrabani, Dehradun 248 001, Uttarakhand, India 4 Corresponding author. E-mail: [email protected] Abstract: A taxonomic inventorization of pteridophytes (Kholia 2014). Generally, it is believed that modern ferns occurring in a human inhabited buffer zone of Murlen and their allies are much older than flowering plants, and National Park, India, was conducted in 2012 and 2013. often considered as living fossils, but except for a few This survey revealed 35 species belonging to 27 genera families, most of the modern ferns evolved and flourished and 15 families. Polypodiaceae was recorded as dominant under the shadow of angiosperms (Smith et al. 2006). family, represented by six genera and eight species, In India, pteridophytes are mainly distributed in Hima- followed by Pteridaceae (three genera and six species) layan region, as well as North-Eastern and Southern India, and Lycopodiaceae (three genera and four species). Of the where climates are humid and more conducive for growth. recorded species, 23 species were terrestrial, 11 (epiphytic) Approximately 1,267 species of pteridophytes (ca. -

Rare and Threatened Pteridophytes of Asia 2. Endangered Species of India — the Higher IUCN Categories
Bull. Natl. Mus. Nat. Sci., Ser. B, 38(4), pp. 153–181, November 22, 2012 Rare and Threatened Pteridophytes of Asia 2. Endangered Species of India — the Higher IUCN Categories Christopher Roy Fraser-Jenkins Student Guest House, Thamel. P.O. Box no. 5555, Kathmandu, Nepal E-mail: [email protected] (Received 19 July 2012; accepted 26 September 2012) Abstract A revised list of 337 pteridophytes from political India is presented according to the six higher IUCN categories, and following on from the wider list of Chandra et al. (2008). This is nearly one third of the total c. 1100 species of indigenous Pteridophytes present in India. Endemics in the list are noted and carefully revised distributions are given for each species along with their estimated IUCN category. A slightly modified update of the classification by Fraser-Jenkins (2010a) is used. Phanerophlebiopsis balansae (Christ) Fraser-Jenk. et Baishya and Azolla filiculoi- des Lam. subsp. cristata (Kaulf.) Fraser-Jenk., are new combinations. Key words : endangered, India, IUCN categories, pteridophytes. The total number of pteridophyte species pres- gered), VU (Vulnerable) and NT (Near threat- ent in India is c. 1100 and of these 337 taxa are ened), whereas Chandra et al.’s list was a more considered to be threatened or endangered preliminary one which did not set out to follow (nearly one third of the total). It should be the IUCN categories until more information realised that IUCN listing (IUCN, 2010) is became available. The IUCN categories given organised by countries and the global rarity and here apply to political India only. -

Botanical Survey of the War in the Pacific National Historical Park Guam, Mariana Islands
PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA Dr. David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 161 Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands July 2008 Joan M. Yoshioka 1 1 Pacific Cooperative Studies Unit (University of Hawai`i at Mānoa), NPS Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawai`i National Park, HI 96718 PCSU is a cooperative program between the University of Hawai`i and U.S. National Park Service, Cooperative Ecological Studies Unit. Organization Contact Information: Inventory and Monitoring Program, Pacific Island Network, PO Box 52, Hawaii National Park, HI 96718, phone: 808-985-6183, fax: 808-985-6111 Recommended Citation: Yoshioka, J. M. 2008. Botanical survey of the War in the Pacific National Historical Park Guam, Mariana Islands. Pacific Cooperative Studies Unit Technical Report 161, University of Hawai`i at Manoa, Department of Botany, Honolulu, HI. Key words: Vegetation types, Vegetation management, Alien species, Endemic species, Checklist, Ferns, Flowering plants Place key words: War in the Pacific National Historical Park, Guam Editor: Clifford W. Morden, PCSU Deputy Director (Mail to: mailto:[email protected]) i Table of Contents List of Tables......................................................................................................iii List of Figures ....................................................................................................iii -

National Wetland Plant List: 2016 Wetland Ratings
Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1–17. Published 28 April 2016. ISSN 2153 733X THE NATIONAL WETLAND PLANT LIST: 2016 WETLAND RATINGS ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, New Hampshire 03755-1290 DARIN L. BANKS U.S. Environmental Protection Agency, Region 7 Watershed Support, Wetland and Stream Protection Section 11201 Renner Boulevard Lenexa, Kansas 66219 WILLIAM N. KIRCHNER U.S. Fish and Wildlife Service, Region 1 911 NE 11 th Avenue Portland, Oregon 97232 NORMAN C. MELVIN USDA Natural Resources Conservation Service Central National Technology Support Center 501 W. Felix Street, Bldg. 23 Fort Worth, Texas 76115-3404 ABSTRACT The U.S. Army Corps of Engineers (Corps) administers the National Wetland Plant List (NWPL) for the United States (U.S.) and its territories. Responsibility for the NWPL was transferred to the Corps from the U.S. Fish and Wildlife Service (FWS) in 2006. From 2006 to 2012 the Corps led an interagency effort to update the list in conjunction with the U.S. Environmental Protection Agency (EPA), the FWS, and the USDA Natural Resources Conservation Service (NRCS), culminating in the publication of the 2012 NWPL. In 2013 and 2014 geographic ranges and nomenclature were updated. This paper presents the fourth update of the list under Corps administration. During the current update, the indicator status of 1689 species was reviewed. A total of 306 ratings of 186 species were changed during the update. -

Ferns and Lycophytes of Mt. Tago Range, Bukidnon, Southern Philippines: Species Richness, Distribution, and Conservation Status
Philippine Journal of Science 149 (3-a): 773-790, October 2020 ISSN 0031 - 7683 Date Received: 22 May 2020 Ferns and Lycophytes of Mt. Tago Range, Bukidnon, Southern Philippines: Species Richness, Distribution, and Conservation Status Fulgent P. Coritico1,2*, Victor B. Amoroso1,2, Florfe M. Acma1,2, Yvonne Love L. Cariño1, and Peter W. Fritsch3 1Center for Biodiversity Research and Extension in Mindanao 2Department of Biology, College of Arts and Sciences Central Mindanao University (CMU), Musuan, Bukidnon 8710 Philippines 3Botanical Research Institute of Texas (BRIT) 1700 University Drive, Fort Worth, TX 76107-3400 USA The species of ferns and lycophytes of Mt. Tago range are here documented in a checklist, along with information on their distribution and conservation status. The list is based on a comprehensive field survey conducted by the authors in 2018 and 2019 and comprises 203 species belonging to 29 families and 89 genera. Of these species, 187 are ferns and 16 are lycophytes. Eighty-six species are epiphytes, 85 are terrestrial, 12 are tree ferns, 6 are hemiepiphytes, and 14 species have more than one growth form. The number of species constitutes about 19% and 33% of the total number of pteridophyte species in the Philippines and Mindanao Island, respectively. The highest species richness was observed in the upper montane rainforest. Seventeen species are classified as broadly distributed Philippine endemics whereas four species are endemic to Mindanao. One species (Alsophila commutata Mett.) was newly documented for the Philippines and three species [Pteris whitfordii Copel., Selliguea elmeri (Copel.) Ching, and Selliguea pyrolifolia (Goldm.) Hovenkamp] were newly documented for Mindanao Island.