Adrenal Cortex 1, 2, & 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

COMMENTARY Possible New Mechanism of Cortisol Action In
211 COMMENTARY Possible new mechanism of cortisol action in female reproductive organs: physiological implications of the free hormone hypothesis C Yding Andersen Laboratory of Reproductive Biology, Section 5712, University Hospital of Copenhagen, DK-2100 Copenhagen, Denmark (Requests for offprints should be addressed to C Yding Andersen; Email: [email protected]) Abstract The so-called free hormone hypothesis predicts that the cortisol to cortisone, while 11-HSD type 1 reverses this biological activity of a given steroid correlates with the free reaction. As a result, a high concentration of cortisol protein-unbound concentration rather than with the total available for biological action is present in the preovulatory concentration (i.e. free plus protein-bound). Cortisol is a follicle just prior to ovulation and it has been suggested that glucocorticoid with many diverse functions and the free cortisol may function to reduce the inflammatory-like hormone hypothesis seems to apply well to the observed reactions occurring in connection with ovulation. effects of cortisol. The ovaries express glucocorticoid This paper suggests (1) that the function of the oviduct receptors and are affected by cortisol, but lack the neces- is also affected by the high levels of free cortisol released in sary enzymes for cortisol synthesis. Ovarian follicles modu- preovulatory follicular fluid at ovulation and (2) that late the biological activity of cortisol by (1) follicular formation and function of the corpus luteum benefits from production of especially progesterone and 17-hydroxy- a high local concentration of free cortisol, whereas the progesterone which, within the follicle, reach levels that surrounding developing follicles may experience negative displace cortisol from its binding proteins, in particular, effects. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
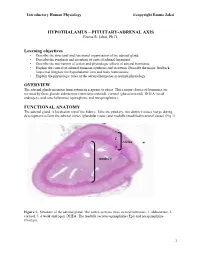
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

Part I Biopharmaceuticals
1 Part I Biopharmaceuticals Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 3 1 Analogs and Antagonists of Male Sex Hormones Robert W. Brueggemeier The Ohio State University, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Columbus, Ohio 43210, USA 1Introduction6 2 Historical 6 3 Endogenous Male Sex Hormones 7 3.1 Occurrence and Physiological Roles 7 3.2 Biosynthesis 8 3.3 Absorption and Distribution 12 3.4 Metabolism 13 3.4.1 Reductive Metabolism 14 3.4.2 Oxidative Metabolism 17 3.5 Mechanism of Action 19 4 Synthetic Androgens 24 4.1 Current Drugs on the Market 24 4.2 Therapeutic Uses and Bioassays 25 4.3 Structure–Activity Relationships for Steroidal Androgens 26 4.3.1 Early Modifications 26 4.3.2 Methylated Derivatives 26 4.3.3 Ester Derivatives 27 4.3.4 Halo Derivatives 27 4.3.5 Other Androgen Derivatives 28 4.3.6 Summary of Structure–Activity Relationships of Steroidal Androgens 28 4.4 Nonsteroidal Androgens, Selective Androgen Receptor Modulators (SARMs) 30 4.5 Absorption, Distribution, and Metabolism 31 4.6 Toxicities 32 Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 4 Analogs and Antagonists of Male Sex Hormones 5 Anabolic Agents 32 5.1 Current Drugs on the Market 32 5.2 Therapeutic Uses and Bioassays -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

PROMETRIUM® (Progesterone, USP) Capsules 200 Mg WARNING
PROMETRIUM® (progesterone, USP) Capsules 100 mg Capsules 200 mg WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY Cardiovascular Disorders and Probable Dementia Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders and Probable dementia.) The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of deep vein thrombosis, pulmonary embolism, stroke and myocardial infarction in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders.) The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS, Probable dementia and PRECAUTIONS, Geriatric Use.) Breast Cancer The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer. (See CLINICAL STUDIES and WARNINGS, Malignant neoplasms, Breast Cancer.) In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins. Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman. -

Oral Contraceptives and Endocrine Changes* 0
Bull. Org. mond. Sante 1972, 46, 443-450 Bull. Wid HIth Org. Oral contraceptives and endocrine changes* 0. J. LUCIS1 & R. LUCIS In groups of women taking oral contraceptives and in control groups ofwomen, the serum levels ofcortisol, protein-bound iodine, and total thyroxine were measured together with the T3 binding index. The daily excretion in the urine offree cortisol, 17-hydroxycorticoste- roids, 17-ketosteroids, pregnanediol, pregnanetriol, total oestrogens, total catecholamines, and 4-hydroxy-3-methoxymandelic acid was also assayed. The frequency distribution of the values obtained indicates that oral contraceptives have a marked influence on the endocrine environment. The smallest deviations were observed in urinary excretion of total catecholamines and of 4-hydroxy-3-methoxymandelic acid. In some individuals the hor- mone assays were continued throughout the menstrual cycle. The morning and afternoon levels of serum cortisol tended to increase during the period when the oral contraceptive was being taken. According to the estimates of the Advisory Com- to prescription, and had been doing so for at least mittee on Obstetrics and Gynecology (1969) of the 2 months. The types of oral contraceptive prepara- United States Food and Drug Administration, tions taken are shown in Table 1. 18.5 million women are using oral contraceptives. The urinary excretion of hormones and their The hormonal balance in these women may show metabolites was determined on 24-hour urine spe- deviations from that seen in normally menstruating cimens. The quantity of free cortisol in the urine was women and this problem was investigated in our assayed by the method of protein displacement bind- laboratory in order to establish the values for com- ing (Murphy, 1967) using newborn calf serum and monly used endocrine assays in women with spon- corticosterone-3H as reagents. -

Summary of Product Characteristics
Prednisolone, DK/H/2488/001-006, March 2021 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT /…/ 2.5 mg tablets /…/ 5 mg tablets /…/ 10 mg tablets /…/ 20 mg tablets /…/ 25 mg tablets /…/ 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2.5 mg prednisolone. Each tablet contains 5 mg prednisolone. Each tablet contains 10 mg prednisolone. Each tablet contains 20 mg prednisolone. Each tablet contains 25 mg prednisolone. Each tablet contains 30 mg prednisolone. Excipient with known effect: Each 2.5 mg tablet contains 89.2 mg of lactose monohydrate Each 5 mg tablet contains 87.2 mg of lactose monohydrate Each 10 mg tablet contains 81.7 mg of lactose monohydrate Each 20 mg tablet contains 163.4 mg of lactose monohydrate Each 25 mg tablet contains 159.4 mg of lactose monohydrate Each 30 mg tablet contains 153.4 mg of lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablets 2.5mg tablet Yellow, 7mm, round, flat, tablet, with a score line on one side, imprinted with “A610” on one side and “2.5” on the other. 5mg tablet White, 7mm, round, flat, tablet, with a score line on one side, imprinted with “A620” on one side and “5” on the other. 10mg tablet Red, 7mm, round, flat, tablet, with a score line on one side, imprinted with “A630” on one side and “10” on the other. 20mg tablet Red, 9mm, round, flat, tablet, with a score line on one side, imprinted with “A640” on one side and “20” on the other. -

LCMS Saliva Steroid & Steroid Synthesis Inhibitor Profile
PROVIDER DATA SHEET LCMS Saliva Steroid & Steroid Synthesis Inhibitor Profile ZRT Laboratory now offers a comprehensive LC-MS/MS saliva assay that measures the levels of 18 endogenous steroid hormones (see Steroid Hormone Cascade Tests Included diagram on the next page) including estrogens, progestogens, androgens, Estrogens glucocorticoids, and mineralocorticoids. In addition to endogenous hormones Estradiol (E2), Estriol (E3), Estrone (E1), the new assay quantifies the level of melatonin and the synthetic estrogen ethinyl Ethinyl Estradiol (EE) estradiol, present in most birth control formulations, as well as several synthetic Progestogen Precursors and Metabolites aromatase inhibitors (anastrozole and letrozole) and the 5α-reductase inhibitor Pregnenolone Sulfate (PregS), Progesterone (Pg), finasteride. Allopregnenolone (AlloP), 17-OH Progesterone (17OHPg) The LC-MS/MS assay expands beyond the 5-steroid panel of parent hormones Androgen Precursors and Metabolites (estradiol, progesterone, testosterone, DHEAS, and cortisol) currently tested Androstenedione (Adione), Testosterone (T), by immunoassay (IA) at ZRT Laboratory. Testing the levels of both upstream Dihydrotestosterone (DHT), DHEA (D), precursors and downstream metabolites of these parent active steroids, listed DHEA-S (DS), 7-Keto DHEA (7keto) above and shown in the diagram on the next page, will help determine which steroid Glucocorticoid Precursors and Metabolites synthesis enzymes are low, overactive, blocked by natural or pharmaceutical 11-Deoxycortisol (11DC) Cortisol