Geochemistry of the Lanthanide Elements Fernando Bea Department of Mineralogy and Petrology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lanthanides & Actinides Notes
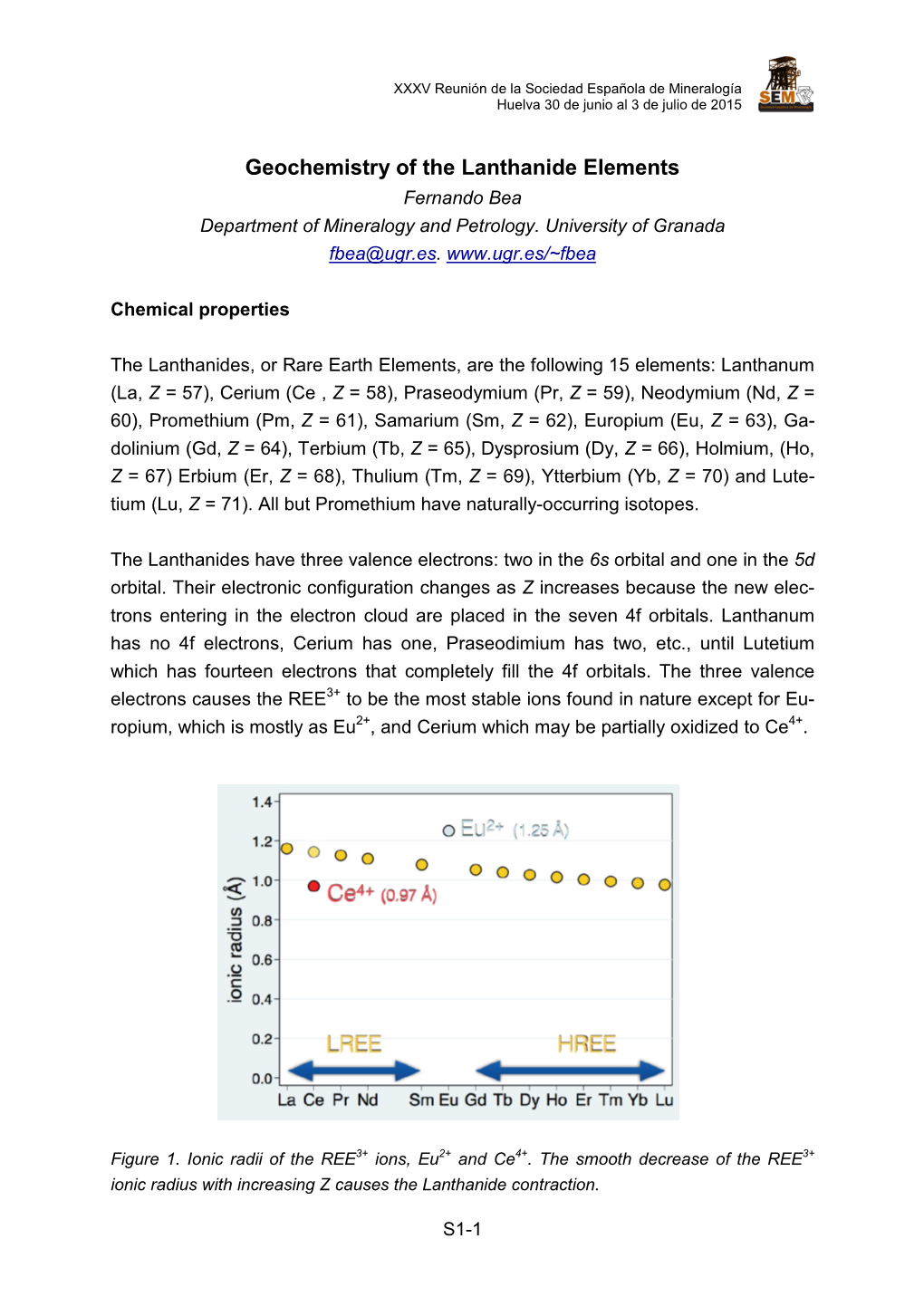
- 1 - LANTHANIDES & ACTINIDES NOTES General Background Mnemonics Lanthanides Lanthanide Chemistry Presents No Problems Since Everyone Goes To Doctor Heyes' Excruciatingly Thorough Yearly Lectures La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Although Theorists Prefer Unusual New Proofs Able Chemists Believe Careful Experiments Find More New Laws Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Principal Characteristics of the Rare Earth Elements 1. Occur together in nature, in minerals, e.g. monazite (a mixed rare earth phosphate). 2. Very similar chemical properties. Found combined with non-metals largely in the 3+ oxidation state, with little tendency to variable valence. 3. Small difference in solubility / complex formation etc. of M3+ are due to size effects. Traversing the series r(M3+) steadily decreases – the lanthanide contraction. Difficult to separate and differentiate, e.g. in 1911 James performed 15000 recrystallisations to get pure Tm(BrO3)3! f-Orbitals The Effective Electron Potential: • Large angular momentum for an f-orbital (l = 3). • Large centrifugal potential tends to keep the electron away from the nucleus. o Aufbau order. • Increased Z increases Coulombic attraction to a larger extent for smaller n due to a proportionately greater change in Zeff. o Reasserts Hydrogenic order. This can be viewed empirically as due to differing penetration effects. Radial Wavefunctions Pn,l2 for 4f, 5d, 6s in Ce 4f orbitals (and the atoms in general) steadily contract across the lanthanide series. Effective electron potential for the excited states of Ba {[Xe] 6s 4f} & La {[Xe] 6s 5d 4f} show a sudden change in the broadness & depth of the 4f "inner well". -

The Periodic Electronegativity Table
The Periodic Electronegativity Table Jan C. A. Boeyens Unit for Advanced Study, University of Pretoria, South Africa Reprint requests to J. C. A. Boeyens. E-mail: [email protected] Z. Naturforsch. 2008, 63b, 199 – 209; received October 16, 2007 The origins and development of the electronegativity concept as an empirical construct are briefly examined, emphasizing the confusion that exists over the appropriate units in which to express this quantity. It is shown how to relate the most reliable of the empirical scales to the theoretical definition of electronegativity in terms of the quantum potential and ionization radius of the atomic valence state. The theory reflects not only the periodicity of the empirical scales, but also accounts for the related thermochemical data and serves as a basis for the calculation of interatomic interaction within molecules. The intuitive theory that relates electronegativity to the average of ionization energy and electron affinity is elucidated for the first time and used to estimate the electron affinities of those elements for which no experimental measurement is possible. Key words: Valence State, Quantum Potential, Ionization Radius Introduction electronegative elements used to be distinguished tra- ditionally [1]. Electronegativity, apart from being the most useful This theoretical notion, in one form or the other, has theoretical concept that guides the practising chemist, survived into the present, where, as will be shown, it is also the most bothersome to quantify from first prin- provides a precise definition of electronegativity. Elec- ciples. In historical context the concept developed in a tronegativity scales that fail to reflect the periodicity of natural way from the early distinction between antag- the L-M curve will be considered inappropriate. -

Additional Teacher Background Chapter 4 Lesson 3, P. 295
Additional Teacher Background Chapter 4 Lesson 3, p. 295 What determines the shape of the standard periodic table? One common question about the periodic table is why it has its distinctive shape. There are actu- ally many different ways to represent the periodic table including circular, spiral, and 3-D. But in most cases, it is shown as a basically horizontal chart with the elements making up a certain num- ber of rows and columns. In this view, the table is not a symmetrical rectangular chart but seems to have steps or pieces missing. The key to understanding the shape of the periodic table is to recognize that the characteristics of the atoms themselves and their relationships to one another determine the shape and patterns of the table. ©2011 American Chemical Society Middle School Chemistry Unit 303 A helpful starting point for explaining the shape of the periodic table is to look closely at the structure of the atoms themselves. You can see some important characteristics of atoms by look- ing at the chart of energy level diagrams. Remember that an energy level is a region around an atom’s nucleus that can hold a certain number of electrons. The chart shows the number of energy levels for each element as concentric shaded rings. It also shows the number of protons (atomic number) for each element under the element’s name. The electrons, which equal the number of protons, are shown as dots within the energy levels. The relationship between atomic number, energy levels, and the way electrons fill these levels determines the shape of the standard periodic table. -

Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System
International Journal of Environmental Science and Development, Vol. 3, No. 4, August 2012 Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System Sabry M. Shaheen, Aly S. Derbalah, and Farahat S. Moghanm rich volcanic rocks (tuff) with fresh water in playa lakes or Abstract—In this study, the sorption behaviour of natural by seawater [5]. (clinoptilolite) zeolites with respect to cadmium (Cd), copper The structures of zeolites consist of three-dimensional (Cu), nickel (Ni), lead (Pb) and zinc (Zn) has been studied in frameworks of SiO and AlO tetrahedra. The aluminum ion order to consider its application to purity metal finishing 4 4 wastewaters. The batch method has been employed, using is small enough to occupy the position in the center of the competitive sorption system with metal concentrations in tetrahedron of four oxygen atoms, and the isomorphous 4+ 3+ solution ranging from 50 to 300 mg/l. The percentage sorption replacement of Si by Al produces a negative charge in and distribution coefficients (Kd) were determined for the the lattice. The net negative charge is balanced by the sorption system as a function of metal concentration. In exchangeable cation (sodium, potassium, or calcium). These addition lability of the sorbed metals was estimated by DTPA cations are exchangeable with certain cations in solutions extraction following their sorption. The results showed that Freundlich model described satisfactorily sorption of all such as lead, cadmium, zinc, and manganese [6]. The fact metals. Zeolite sorbed around 32, 75, 28, 99, and 59 % of the that zeolite exchangeable ions are relatively innocuous added Cd, Cu, Ni, Pb and Zn metal concentrations (sodium, calcium, and potassium ions) makes them respectively. -

Electronic Structure of Ytterbium(III) Solvates – a Combined Spectro
Electronic Structure of Ytterbium( III) Solvates – A Combined Spectro- scopic and Theoretical Study Nicolaj Kofod,† Patrick Nawrocki,† Carlos Platas-Iglesias,*,‡ Thomas Just Sørensen*,† † Department of Chemistry and Nano-Science Center, University of Copenhagen, Universitetsparken 5, 2100 København Ø, Den- mark. ‡ Centro de Investigacións Científicas Avanzadas and Departamento de Química, Universidade da Coruña, Campus da Zapateira-Rúa da Fraga 10, 15008 A Coruña, Spain ABSTRACT: The wide range of optical and magnetic properties of the lanthanide(III) ions is associated to their intricate electronic structures, which in contrast to lighter elements is characterized by strong relativistic effects and spin-orbit coupling. Nevertheless, computational methods are now capable of describing the ladder of electronic energy levels of the simpler trivalent lanthanide ions, as well as the lowest energy term of most of the series. The electronic energy levels result from electron configurations that are first split by spin-orbit coupling into groups of energy levels denoted by the corresponding Russel-Saunders terms. Each of these groups are then split by the ligand field into the actual electronic energy levels known as microstates or sometimes mJ levels. The ligand field splitting directly informs on coordination geometry, and is a valuable tool for determining structure and thus correlating the structure and properties of metal complexes in solution. The issue with lanthanide com- plexes is that the determination of complex structures from ligand field splitting remains a very challenging task. In this manuscript, the optical spectra – absorption, luminescence excitation and luminescence emission – of ytterbium(III) solvates were recorded in water, methanol, dime- thyl sulfoxide and N,N-dimethylformamide. -

Why Isn't Hafnium a Noble Gas? Also More on the Lanthanide Contraction
return to updates Period 6 Why Isn't Hafnium a Noble Gas? also more on the Lanthanide contraction by Miles Mathis First published March 30, 2014 This paper replaces my earlier paper on the Lanthanides After a long break, it is time I returned to the Periodic Table. Many readers probably wish I would concentrate more on one subject, or at least one area of physics or chemistry. Possibly, they think I would get more done that way. They are mistaken. I would indeed get more done in that one field, and if that is their field, of course that would satisfy them more personally. But by skipping around, I actually maximize my production. How? One, I stay fresh. I don't get bored by staying in one place too long, so my creativity stays at a peak. Two, I cross-pollinate my ideas. My readers have seen how often a discovery in one sub-field helps me in another sub-field, even when those two fields aren't adjacent. All of science (and life) is ultimately of a piece, so anything I learn anywhere will help me everywhere else. Three, being interested in a wide array of topics gives me a bigger net, and with a bigger net I am better able to capture solutions across the board. Knowledge isn't just a matter of depth, it is a matter of breadth. In philosophy classes, we were taught this as part of the hermeneutic circle: the parts feed the whole and the whole feeds back into all the parts. -

Most Atoms Form Chemical Bonds to Obtain a Lower Potential Energy
Integrated Chemistry <Kovscek> 6-1-2 Explain why most atoms form chemical bonds. Most atoms form chemical bonds to obtain a lower potential energy. Many share or transfer electrons to get a noble gas configuration. 6-1-3 Describe ionic and covalent bonding. Ions form to lower the reactivity of an atom. Ionic bonding is when the attraction between many cations and anions hold atoms together. Covalent bonding results from the sharing of electron pairs between two atoms. That is, when atoms share electrons, the mutual attraction between the electrons (electron clouds) and protons (the nucleus) hold the atoms together. 6-1-4 Explain why most chemical bonding is neither purely ionic nor purely covalent. In order for a bond to be totally ionic one atom would have to have an electronegativity of zero. (there aren’t any). To have a purely covalent bond the electronegativity must be equal. (Only the electronegativity of atoms of the same element are identical, diatomic elements are purely covalent) 6-1-5 Classify bonding type according to electronegativity differences. Differences in electronegativities reflect the character of bonding between elements. The electronegativity of the less- electronegative element is subtracted from that of the more- electronegative element. The greater the electronegativity difference, the more ionic is the bonding. Covalent Bonding happens between atoms with an electronegativity difference of 1.7 or less. That is the bond has an ionic character of 50% or less. Electronegativity % Ionic Character Covalent Differences Bond Type 0 to 0.3 0% to 5% Non - Polar Over 0.3 up to 1.7 5% to 50% Polar Over 1.7 Greater than 50 % Ionic Ionic Bonding occurs between atoms with an electronegativity difference of greater than 1.7. -

ELECTRONEGATIVITY D Qkq F=
ELECTRONEGATIVITY The electronegativity of an atom is the attracting power that the nucleus has for it’s own outer electrons and those of it’s neighbours i.e. how badly it wants electrons. An atom’s electronegativity is determined by Coulomb’s Law, which states, “ the size of the force is proportional to the size of the charges and inversely proportional to the square of the distance between them”. In symbols it is represented as: kq q F = 1 2 d 2 where: F = force (N) k = constant (dependent on the medium through which the force is acting) e.g. air q1 = charge on an electron (C) q2 = core charge (C) = no. of protons an outer electron sees = no. of protons – no. of inner shell electrons = main Group Number d = distance of electron from the nucleus (m) Examples of how to calculate the core charge: Sodium – Atomic number 11 Electron configuration 2.8.1 Core charge = 11 (no. of p+s) – 10 (no. of inner e-s) +1 (Group i) Chlorine – Atomic number 17 Electron configuration 2.8.7 Core charge = 17 (no. of p+s) – 10 (no. of inner e-s) +7 (Group vii) J:\Sciclunm\Resources\Year 11 Chemistry\Semester1\Notes\Atoms & The Periodic Table\Electronegativity.doc Neon holds onto its own electrons with a core charge of +8, but it can’t hold any more electrons in that shell. If it was to form bonds, the electron must go into the next shell where the core charge is zero. Group viii elements do not form any compounds under normal conditions and are therefore given no electronegativity values. -

Schematic Interpretation of Anomalies in the Physical Properties of Eu and Yb Among the Lanthanides
International Journal of Materials Science and Applications 2017; 6(4): 165-170 http://www.sciencepublishinggroup.com/j/ijmsa doi: 10.11648/j.ijmsa.20170604.11 ISSN: 2327-2635 (Print); ISSN: 2327-2643 (Online) Schematic Interpretation of Anomalies in the Physical Properties of Eu and Yb Among the Lanthanides Yoshiharu Mae Maetech, Mimuro, Midori Ward, Saitama City, Japan Email address: [email protected] To cite this article: Yoshiharu Mae. Schematic Interpretation of Anomalies in the Physical Properties of Eu and Yb Among the Lanthanides. International Journal of Materials Science and Applications. Vol. 6, No. 4, 2017, pp. 165-170. doi: 10.11648/j.ijmsa.20170604.11 Received: May 24, 2017; Accepted: June 2, 2017; Published: June 19, 2017 Abstract: Lanthanides are the elements in 6th period and the 3rd group of the periodic table. Eu and Yb exhibit some unusual properties compared with the other lanthanides. The author has proposed a diagram to systematically illustrate the properties of the elements, by plotting the Young’s modulus on the ordinate and thermal conductivity on the abscissa. Eu and Yb have much lower Young’s moduli, and are located far from other lanthanides on the diagram. Most lanthanides have hexagonal structures. Eu, however, has a body-centered cubic structure, because it is located on the extension of the curve of alkali metals. Yb has a face-centered cubic (fcc) structure, because it is located on the curve of fcc metals. The positions of Eu and Yb on the diagram are thought to act as a bridge between the lanthanides and other adjacent element groups. -

Lanthanides.Pdf
Lanthanides [A] LANTHANIDES : 4f block elements Definition: The f- block (inner transition) elements containing partially filled 4f-subshells are known as Lanthanides or Lanthanones because of their close similarities with element lanthanum (atomic no: 57). The fourteen elements from atomic no: 58 to 71 constitute lanthanides. Nos. Name Symbol Electronic configuration 0 1 2 1. Lanthanum La57 [Xe] 4f 5d 6s 2 0 2 2. Cerium Ce58 [Xe] 4f 5d 6s 3 0 2 3. Praseodymium Pr59 [Xe] 4f 5d 6s 4 0 2 4. Neodymium Nd60 [Xe] 4f 5d 6s 5 0 2 5. Promethium Pm61 [Xe]4f 5d 6s 6 0 2 6. Samarium Sm62 [Xe]4f 5d 6s 7 0 2 7. Europium Eu63 [Xe] 4f 5d 6s 7 1 2 8. Gadolinium Gd64 [Xe] 4f 5d 6s 9 0 2 9. Terbium Tb65 [Xe] 4f 5d 6s 10 0 2 10. Dysprosium Dy66 [Xe] 4f 5d 6s 11 0 2 11. Holmium Ho67 [Xe] 4f 5d 6s 12 0 2 12. Erbium Er68 [Xe] 4f 5d 6s 13 0 2 13. Thulium Tm69 [Xe] 4f 5d 6s 14 0 2 14. Ytterbium Yb70 [Xe] 4f 5d 6s 14 1 2 15. Lutetium Lu71 [Xe] 4f 5d 6s From the above electronic configuration it can be seen that at La 5d orbital is singly occupied but after La further filling of 5d orbital is discontinued. As the nuclear charge increases by one unit from La to Ce, 4f orbitals were higher in energy upto Lu, fall slightly below the 5d level 4f- orbitals, therefore begin to fill and are completely filled up to Lu, before filling of 5d orbital is resumed. -

Periodic Table of the Elements Notes
Periodic Table of the Elements Notes Arrangement of the known elements based on atomic number and chemical and physical properties. Divided into three basic categories: Metals (left side of the table) Nonmetals (right side of the table) Metalloids (touching the zig zag line) Basic Organization by: Atomic structure Atomic number Chemical and Physical Properties Uses of the Periodic Table Useful in predicting: chemical behavior of the elements trends properties of the elements Atomic Structure Review: Atoms are made of protons, electrons, and neutrons. Elements are atoms of only one type. Elements are identified by the atomic number (# of protons in nucleus). Energy Levels Review: Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms Energy Levels & Valence Electrons Energy levels hold a specific amount of electrons: 1st level = up to 2 2nd level = up to 8 3rd level = up to 8 (first 18 elements only) The electrons in the outermost level are called valence electrons. Determine reactivity - how elements will react with others to form compounds Outermost level does not usually fill completely with electrons Using the Table to Identify Valence Electrons Elements are grouped into vertical columns because they have similar properties. These are called groups or families. Groups are numbered 1-18. Group numbers can help you determine the number of valence electrons: Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Groups 3–12 are transition metals and have 1 or 2 valence electrons. -

Lanthanide Contraction Lanthanide Contraction W Urood A
Lanthanide contraction Wurood A. Jaafar, Enass J. Waheed, Zainab A. Jabarah* Department of Chemistry, College of Education for Pure Science (Ibn Al-Haitham), University of Baghdad, Baghdad, Iraq. *Basic Science Division, College of Agriculture Engineering Science, University of Baghdad, Baghdad, Iraq. The lanthanides-or lanthanids, as they are sometimes called- are, strictly, the fourteen elements that follow lanthanum in the periodic table and in which the fourteen 4f electrons are successively added to the lanthanum configuration. Since these 4f relatively uninvolved in bonding, the main result is that all these highly electropositive elements have; as their prime oxidation state, the M+3 ion. The lanthanide series consist of the 14 elements, with atomic numbers 58 through 71…… Lanthanide contraction was used in discussing the elements of the third transition series, since it has certain important effects on their properties. It consists of a significant and steady decrease in the size of the atoms and ions with increasing atomic number, that is, La has the greatest, and Lu the smallest radius. The effect results from poor shielding of nuclear charge (nuclear attractive force on electrons) by 4f electrons; the 6s electrons are drawn towards the nucleus, thus resulting in a smaller atomic radius. In single-electron atoms, the average separation of an electron from the nucleus is determined by the subshell it belongs to, and decreases with increasing charge on the nucleus; this in turn leads to a decrease in atomic radius. In multi-electron atoms, the decrease in radius brought about by an increase in nuclear charge is partially offset by increasing electrostatic repulsion among electrons.