The Cranium of Parapithecus Grangeri, an Egyptian Oligocene Anthropoidean Primate
Total Page:16
File Type:pdf, Size:1020Kb
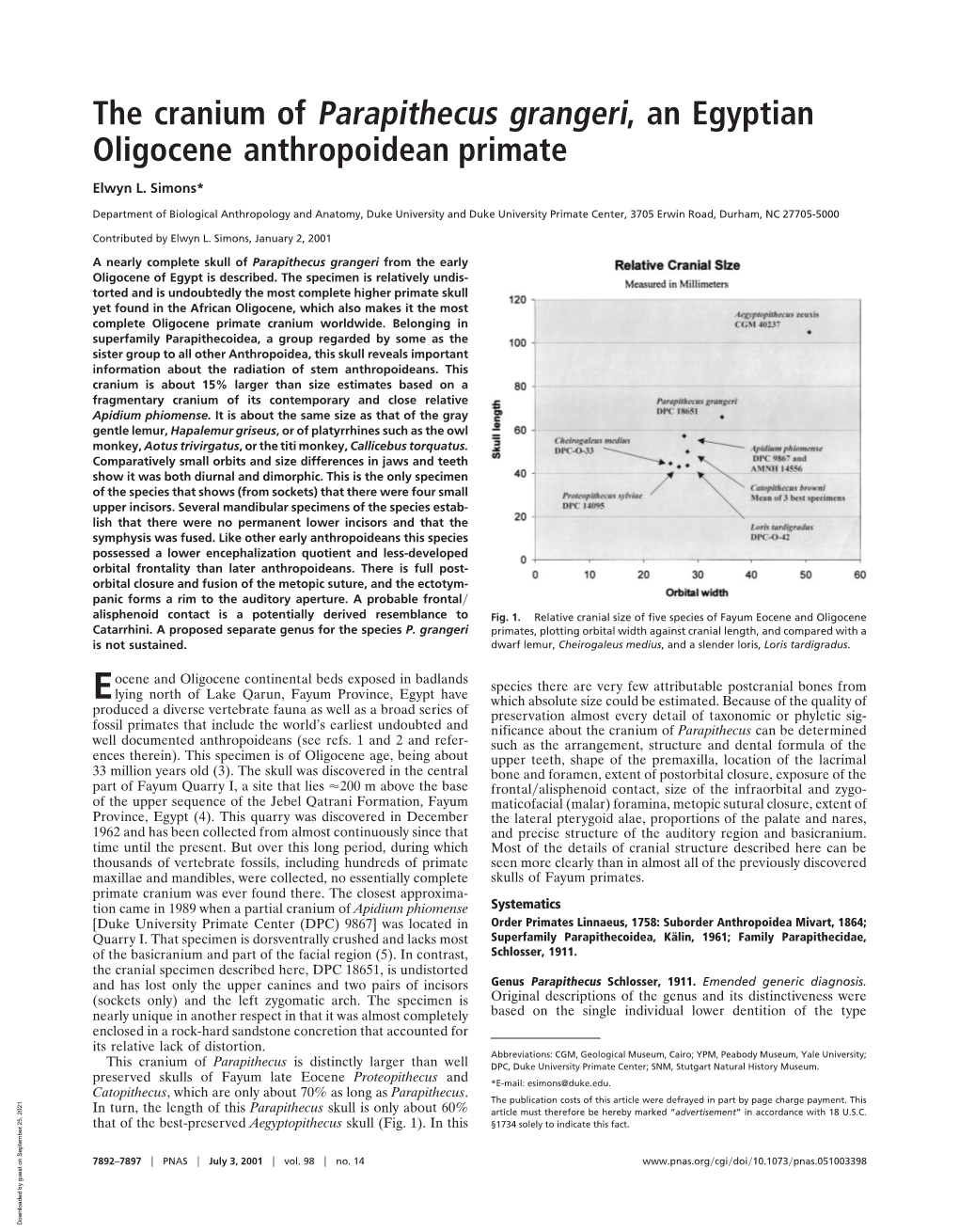
Load more
Recommended publications
-

The Cat Mandible (II): Manipulation of the Jaw, with a New Prosthesis Proposal, to Avoid Iatrogenic Complications
animals Review The Cat Mandible (II): Manipulation of the Jaw, with a New Prosthesis Proposal, to Avoid Iatrogenic Complications Matilde Lombardero 1,*,† , Mario López-Lombardero 2,†, Diana Alonso-Peñarando 3,4 and María del Mar Yllera 1 1 Unit of Veterinary Anatomy and Embryology, Department of Anatomy, Animal Production and Clinical Veterinary Sciences, Faculty of Veterinary Sciences, Campus of Lugo—University of Santiago de Compostela, 27002 Lugo, Spain; [email protected] 2 Engineering Polytechnic School of Gijón, University of Oviedo, 33203 Gijón, Spain; [email protected] 3 Department of Animal Pathology, Faculty of Veterinary Sciences, Campus of Lugo—University of Santiago de Compostela, 27002 Lugo, Spain; [email protected] 4 Veterinary Clinic Villaluenga, calle Centro n◦ 2, Villaluenga de la Sagra, 45520 Toledo, Spain * Correspondence: [email protected]; Tel.: +34-982-822-333 † Both authors contributed equally to this manuscript. Simple Summary: The small size of the feline mandible makes its manipulation difficult when fixing dislocations of the temporomandibular joint or mandibular fractures. In both cases, non-invasive techniques should be considered first. When not possible, fracture repair with internal fixation using bone plates would be the best option. Simple jaw fractures should be repaired first, and caudal to rostral. In addition, a ventral approach makes the bone fragments exposure and its manipulation easier. However, the cat mandible has little space to safely place the bone plate screws without damaging the tooth roots and/or the mandibular blood and nervous supply. As a consequence, we propose a conceptual model of a mandibular prosthesis that would provide biomechanical Citation: Lombardero, M.; stabilization, avoiding any unintended (iatrogenic) damage to those structures. -

8. Primate Evolution
8. Primate Evolution Jonathan M. G. Perry, Ph.D., The Johns Hopkins University School of Medicine Stephanie L. Canington, B.A., The Johns Hopkins University School of Medicine Learning Objectives • Understand the major trends in primate evolution from the origin of primates to the origin of our own species • Learn about primate adaptations and how they characterize major primate groups • Discuss the kinds of evidence that anthropologists use to find out how extinct primates are related to each other and to living primates • Recognize how the changing geography and climate of Earth have influenced where and when primates have thrived or gone extinct The first fifty million years of primate evolution was a series of adaptive radiations leading to the diversification of the earliest lemurs, monkeys, and apes. The primate story begins in the canopy and understory of conifer-dominated forests, with our small, furtive ancestors subsisting at night, beneath the notice of day-active dinosaurs. From the archaic plesiadapiforms (archaic primates) to the earliest groups of true primates (euprimates), the origin of our own order is characterized by the struggle for new food sources and microhabitats in the arboreal setting. Climate change forced major extinctions as the northern continents became increasingly dry, cold, and seasonal and as tropical rainforests gave way to deciduous forests, woodlands, and eventually grasslands. Lemurs, lorises, and tarsiers—once diverse groups containing many species—became rare, except for lemurs in Madagascar where there were no anthropoid competitors and perhaps few predators. Meanwhile, anthropoids (monkeys and apes) emerged in the Old World, then dispersed across parts of the northern hemisphere, Africa, and ultimately South America. -

Why Fuse the Mandibular Symphysis? a Comparative Analysis
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 112:517–540 (2000) Why Fuse the Mandibular Symphysis? A Comparative Analysis D.E. LIEBERMAN1,2 AND A.W. CROMPTON2 1Department of Anthropology, George Washington University, Washington, DC 20052, and Human Origins Program, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 2Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 02138 KEY WORDS symphysis; mammals; primates; electromyograms; mandible; mastication ABSTRACT Fused symphyses, which evolved independently in several mammalian taxa, including anthropoids, are stiffer and stronger than un- fused symphyses. This paper tests the hypothesis that orientations of tooth movements during occlusion are the primary basis for variations in symph- yseal fusion. Mammals whose teeth have primarily dorsally oriented occlusal trajectories and/or rotate their mandibles during occlusion will not benefit from symphyseal fusion because it prevents independent mandibular move- ments and because unfused symphyses transfer dorsally oriented forces with equal efficiency; mammals with predominantly transverse power strokes are predicted to benefit from symphyseal fusion or greatly restricted mediolateral movement at the symphysis in order to increase force transfer efficiency across the symphysis in the transverse plane. These hypotheses are tested with comparative data on symphyseal and occlusal morphology in several mammals, and with kinematic and EMG analyses of mastication in opossums (Didelphis virginiana) and -

Identification Notes &~@~-/~: ~~*~@~,~ 'PTILE
CATEGORY Identification Notes &~@~-/~: ~~*~@~,~ ‘PTILE for wildlife law enforcement ~ C.rnrn.n N.rn./s: Al@~O~, c~~~dil., ~i~.xl, Gharial PROBLEM: Skulls of Crocodilians are often imported as souvenirs. nalch (-”W 4(JI -“by ieeth ??la&ularJy+i9 GUIDE TO PRELIMINARY IDENTIFICATION OF CROCODILL4N SKULLS 1. Nasal bones separated from nasal aperture; mandibular symphysis extends to the 15th tooth. 2. Gavialis gangeticus Nasal bones entering the nasal aperture; mandibular symphysisdoes not extend beyond the8th tooth . Tomistoma schlegelii 2. Nasal bones separated from premaxillary bones; 27 -29maxi11aryteeth,25 -26mandibularteeth Nasal bones in contact with premaxillaq bo Qoco@khs acutus teeth, 18-19 mandibular teeth . Tomiitomaschlegelii 3. Fourth mandibular tooth usually fitting into a notch in the maxilla~, 16-19 maxillary teeth, 14-15 mandibular teeth . .4 Osteolaemus temaspis Fourth mandibular tooth usually fitting into a pit in the maxilla~, 14-20 maxillary teeth, 17-22 mandibular teeth . .5 4. Nasal bones do not divide nasal aperture. .. CrocodylW (12 species) Alligator m&siss@piensh Nasalboncx divide nasal aperture . Osteolaemustetraspk. 5. Nasal bones do not divide nasal aperture. .6 . Paleosuchus mgonatus Bony septum divides nasal aperture . .. Alligator (2 species) 6. Fiveteethinpremaxilla~ bone . .7 . Melanosuchus niger Four teeth in premaxillary bone. ...Paleosuchus (2species) 7. Vomerexposed on the palate . Melanosuchusniger Caiman crocodiles Vomer not exposed on palate . ...”..Caiman (2species) Illustrations from: Moo~ C. C 1921 Me&m, F. 19S1 L-.. Submitted by: Stephen D. Busack, Chief, Morphology Section, National Fish& Wildlife Forensics LabDate submitted 6/3/91 Prepared in cooperation with the National Fkh & Wdlife Forensics Laboratoy, Ashlar@ OR, USA ‘—m More on reverse side>>> IDentMcation Notes CATEGORY: REPTILE for wildlife law enforcement -- Crocodylia II CAmmom Nda Alligator, Crocodile, Caiman, Gharial REFERENCES Medem, F. -

Illustrated Review of the Embryology and Development of the Facial
REVIEW ARTICLE Illustrated Review of the Embryology and Development of the Facial Region, Part 2: Late Development of the Fetal Face and Changes in the Face from the Newborn to Adulthood P.M. Som and T.P. Naidich ABSTRACT SUMMARY: The later embryogenesis of the fetal face and the alteration in the facial structure from birth to adulthood have been reviewed. Part 3 of the review will address the molecular mechanisms that are responsible for the changes described in parts 1 and 2. art 1 of this 3-part review primarily dealt with the early em- first make contact, each is completely covered by a homoge- Pbryologic development of the face and nasal cavity. Part 2 will neous epithelium. A special epithelium arises at the edge of discuss the later embryonic and fetal development of the face, and each palatal shelf, facilitating the eventual fusion of these changes in facial appearance from neonate to adulthood will be shelves. The epithelium on the nasal cavity surface of the palate reviewed. will differentiate into columnar ciliated epithelium. The epi- thelium on the oral cavity side of the palate will differentiate Formation of the Palate into stratified squamous epithelium. Between the sixth and 12th weeks, the palate is formed from 3 The 2 palatal shelves also fuse with the triangular primary pal- primordia: a midline median palatine process and paired lateral ate anteromedially to form a y-shaped fusion line. The point of palatine processes (Fig 1). In the beginning of the sixth week, fusion of the secondary palatal shelves with the primary palate is merging of the paired medial nasal processes forms the intermax- marked in the adult by the incisive foramen. -

Variation in Chin and Mandibular Symphysis Size and Shape in Males and Females: a CT-Based Study
International Journal of Environmental Research and Public Health Article Variation in Chin and Mandibular Symphysis Size and Shape in Males and Females: A CT-Based Study Tatiana Sella Tunis 1,2,3,* , Israel Hershkovitz 1,2 , Hila May 1,2, Alexander Dan Vardimon 3, Rachel Sarig 2,3,4 and Nir Shpack 3 1 Department of Anatomy and Anthropology, Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv 69978, Israel; [email protected] (I.H.); [email protected] (H.M.) 2 Dan David Center for Human Evolution and Biohistory Research, Shmunis Family Anthropology Institute, Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv 69978, Israel; [email protected] 3 Department of Orthodontics, The Maurice and Gabriela Goldschleger School of Dental Medicine, Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv 69978, Israel; [email protected] (A.D.V.); [email protected] (N.S.) 4 Department of Oral Biology, The Maurice and Gabriela Goldschleger School of Dental Medicine, Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv 69978, Israel * Correspondence: [email protected]; Tel.: +972-3-640-7310 Received: 12 May 2020; Accepted: 11 June 2020; Published: 14 June 2020 Abstract: The chin is a unique anatomical landmark of modern humans. Its size and shape play an important role from the esthetic perspective. However, disagreement exists in the dental and anthropological literature regarding the sex differences in chin and symphysis morphometrics. The “sexual selection” theory is presented as a possible reason for chin formation in our species; however, many other contradictory theories also exist. -

Evidence for an Asian Origin of Stem Anthropoids
Evidence for an Asian origin of stem anthropoids Richard F. Kay1 Department of Evolutionary Anthropology, Duke University, Durham, NC 27708-03083 n PNAS, Chaimanee et al. (1) report Genetic, embryological, and anatomical We are not there yet. Recent comprehen- a previously undescribed species of evidence demonstrates that the sister sive phylogenetic analyses stemming from I primate, Afrasia, from the late Middle group of Anthropoidea is the south Asian virtually the same datasets yield somewhat Eocene of Burma. They identify tarsier, with the two forming the crown different cladograms that reflect sensitivity Afrasia as the sister taxon to the African group Haplorhini (6–8). The other clade of to which taxa are included in the analysis genus Afrotarsius but slightly more primitive extant primates is the lemurs and lorises, and which sets of analytical assumptions are than it and allied with stem Anthropoidea called Strepsirrhini. Eocene Holarctic selected. Pertinent to the biogeographic of south Asia. Anthropoidea is the taxo- Omomyoidea are generally considered as conclusions of Chaimanee et al. (1), Seiffert nomic group that today includes New and stem haplorhines, although the precise et al. (11) conclude that Afrotarsius cha- Old World monkeys, apes, and humans. If relationship of omomyoids to tarsiers and trathi [a younger species than the one that upheld, the biogeographic significance of anthropoids is uncertain (8). Tarsius often Chaimanee et al. (1) describe] is an African these results is profound: If Afrasia and is considered to be a relictual omomyoid tarsioid. If Seiffert et al. (11) are correct, Afrotarsius are as closely related as Chai- in south Asia. -

1 Old World Monkeys
2003. 5. 23 Dr. Toshio MOURI Old World monkey Although Old World monkey, as a word, corresponds to New World monkey, its taxonomic rank is much lower than that of the New World Monkey. Therefore, it is speculated that the last common ancestor of Old World monkeys is newer compared to that of New World monkeys. While New World monkey is the vernacular name for infraorder Platyrrhini, Old World Monkey is the vernacular name for superfamily Cercopithecoidea (family Cercopithecidae is limited to living species). As a side note, the taxon including Old World Monkey at the same taxonomic level as New World Monkey is infraorder Catarrhini. Catarrhini includes Hominoidea (humans and apes), as well as Cercopithecoidea. Cercopithecoidea comprises the families Victoriapithecidae and Cercopithecidae. Victoriapithecidae is fossil primates from the early to middle Miocene (15-20 Ma; Ma = megannum = 1 million years ago), with known genera Prohylobates and Victoriapithecus. The characteristic that defines the Old World Monkey (as synapomorphy – a derived character shared by two or more groups – defines a monophyletic taxon), is the bilophodonty of the molars, but the development of biphilophodonty in Victoriapithecidae is still imperfect, and crista obliqua is observed in many maxillary molars (as well as primary molars). (Benefit, 1999; Fleagle, 1999) Recently, there is an opinion that Prohylobates should be combined with Victoriapithecus. Living Old World Monkeys are all classified in the family Cercopithecidae. Cercopithecidae comprises the subfamilies Cercopithecinae and Colobinae. Cercopithecinae has a buccal pouch, and Colobinae has a complex, or sacculated, stomach. It is thought that the buccal pouch is an adaptation for quickly putting rare food like fruit into the mouth, and the complex stomach is an adaptation for eating leaves. -
CHAPTER 9 - LOWER PRIMATES and MONKEYS We Humans Are Classified As Members of Order Primates
CHAPTER 9 - LOWER PRIMATES AND MONKEYS We humans are classified as members of Order Primates. In the Linnaean system, Primates are commonly broken down into subgroups as follows. Visual ORDER PRIMATES # 9-1 Suborder Prosimii Suborder Anthropoidea (Strepsirrhines) (Haplorrhines) Lemurs/Lorises Aye-ayes Adapoids Tarsiers Omomyids Platyrrhines Catarrhines (extinct) (extinct) (nostrils nostrils facing facing sideways) down or front) New World Old World Apes Humans monkeys monkeys (after Perelman et al., 2011) There are obviously similarities between humans, monkeys, and apes. There are contradictory explanations: (1) Common design, and (2) Common ancestry with later evolution. Each is a matter of faith and cannot be tested. Initial Complexity leads us to believe that humans and each primate kind were directly created Visual # 9-2 and have varied only within definite genetic limits. By contrast, Initial Disorganization leads us to believe that each type of primate has evolved from some lower type, starting with something similar to lagomorphs (rabbits and hares). Perelman, Johnson et al. (2011) give the most common scenario, that one of these evolved to something like tree shrews (Scandentia), which in turn evolved into Dermoptera (colugos or “flying lemurs”), then primates. In order for us to tell which is more likely to be correct, we can look at the available fossils. • If Initial Complexity is correct, there should be a complete absence of transitional forms between any of the groups shown above. There should also be a resistance to basic change in each of the primate groups. They may exhibit variation within a kind, but there should be no traces of evolution from one kind to another. -

Rapid Laurasian Diversification of a Pantropical Bird Family During The
Ibis (2020), 162, 137–152 doi: 10.1111/ibi.12707 Rapid Laurasian diversification of a pantropical bird family during the Oligocene–Miocene transition CARL H. OLIVEROS,1,2* MICHAEL J. ANDERSEN,3 PETER A. HOSNER,4,7 WILLIAM M. MAUCK III,5,6 FREDERICK H. SHELDON,2 JOEL CRACRAFT5 & ROBERT G. MOYLE1 1Department of Ecology and Evolutionary Biology and Biodiversity Institute, University of Kansas, Lawrence, KS, USA 2Museum of Natural Science and Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA 3Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, NM, USA 4Division of Birds, Department of Vertebrate Zoology, National Museum of Natural History, Washington, DC, USA 5Department of Ornithology, American Museum of Natural History, New York, NY, USA 6New York Genome Center, New York, NY, USA 7Natural History Museum of Denmark and Center for Macroecology, Evolution, and Climate; University of Copenhagen, Copenhagen, Denmark Disjunct, pantropical distributions are a common pattern among avian lineages, but dis- entangling multiple scenarios that can produce them requires accurate estimates of his- torical relationships and timescales. Here, we clarify the biogeographical history of the pantropical avian family of trogons (Trogonidae) by re-examining their phylogenetic rela- tionships and divergence times with genome-scale data. We estimated trogon phylogeny by analysing thousands of ultraconserved element (UCE) loci from all extant trogon gen- era with concatenation and coalescent approaches. We then estimated a time frame for trogon diversification using MCMCTree and fossil calibrations, after which we performed ancestral area estimation using BioGeoBEARS. We recovered the first well-resolved hypothesis of relationships among trogon genera. -

Printing 3D Models of Canine Jaw Fractures for Teaching Undergraduate Veterinary Medicine1
ACTA CIRÚRGICA BRASILEIRA EDUCATION Printing 3D models of canine jaw fractures for teaching undergraduate veterinary medicine1 Agnes de Souza LimaI , Marcello MachadoII , Rita de Cassia Ribeiro PereiraIII , Yuri Karaccas de Carvalho I M.Sc., Postgraduate Program in Health and Animal Production, Universidade Federal do Acre (UFAC), Rio Branco-AC, Brazil. Acquisition, analysis and interpretation of data; manuscript preparation and writing. II D.Sc., Department of Anatomy, Universidade Federal do Paraná (UFPR), Curitiba-PR, Brazil. Scientific and intellectual content of the study. III M.Sc., Health and Sports Science Center, UFAC, Rio Branco-AC, Brazil. Technical procedures. IV D.Sc., Biological and Natural Sciences Center, UFAC, Rio Branco-AC, Brazil. Manuscript writing, critical revision, final approval. Abstract Purpose: To develop 3D anatomical models, and corresponding radiographs, of canine jaw fractures. Methods: A base model was generated from a mandibular bone scan. With this model it was possible to perform fracture planning according to the anatomical location. Results: The 3D base model of the canine mandible was similar in conformation to the natural bone, demonstrating structures such as canine tooth crowns, premolars and molars, mental foramina, body of the mandible, ramus of the mandible, masseteric fossa, the coronoid process, condylar process, and angular process. It was not possible to obtain detail of the crown of the incisor teeth, mandibular symphysis, and the medullary channel. Production of the 3D CJF model took 10.6 h, used 150.1 g of filament (ABS) and cost US$5.83. Conclusion: The 3D canine jaw fractures models, which reproduced natural canine jaw fractures, and their respective radiographic images, are a possible source of educational material for the teaching of veterinary medicine. -

30 Tejedor.Pmd
Arquivos do Museu Nacional, Rio de Janeiro, v.66, n.1, p.251-269, jan./mar.2008 ISSN 0365-4508 THE ORIGIN AND EVOLUTION OF NEOTROPICAL PRIMATES 1 (With 4 figures) MARCELO F. TEJEDOR 2 ABSTRACT: A significant event in the early evolution of Primates is the origin and radiation of anthropoids, with records in North Africa and Asia. The New World Primates, Infraorder Platyrrhini, have probably originated among these earliest anthropoids morphologically and temporally previous to the catarrhine/platyrrhine branching. The platyrrhine fossil record comes from distant regions in the Neotropics. The oldest are from the late Oligocene of Bolivia, with difficult taxonomic attribution. The two richest fossiliferous sites are located in the middle Miocene of La Venta, Colombia, and to the south in early to middle Miocene sites from the Argentine Patagonia and Chile. The absolute ages of these sedimentary deposits are ranging from 12 to 20 Ma, the oldest in Patagonia and Chile. These northern and southern regions have a remarkable taxonomic diversity and several extinct taxa certainly represent living clades. In addition, in younger sediments ranging from late Miocene through Pleistocene, three genera have been described for the Greater Antilles, two genera in eastern Brazil, and at least three forms for Río Acre. In general, the fossil record of South American primates sheds light on the old radiations of the Pitheciinae, Cebinae, and Atelinae. However, several taxa are still controversial. Key words: Neotropical Primates. Origin. Evolution. RESUMO: Origem e evolução dos primatas neotropicais. Um evento significativo durante o início da evolução dos primatas é a origem e a radiação dos antropóides, com registros no norte da África e da Ásia.