Diffuse Panbronchiolitis in Rheumatoid Arthritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diffuse Panbronchiolitis Is Not Restricted to East Asia—A Mini Literature Review
Review Interstitial Lung Disease Diffuse Panbronchiolitis is not Restricted to East Asia—a Mini Literature Review Ram Kumar Mishra Epidemiology and HEOR Team, ODC 3, Tata Consultancy Services, Thane (W), Maharashtra, India DOI: https://doi.org/10.17925/USRPD.2017.12.02.30 iffuse panbronchiolitis (DPB) is a rare inflammatory lung disease, and is well recognized in East Asian countries like Japan, China, Taiwan and Korea. Over the years, sporadic DPB cases have been reported worldwide from other countries. This literature review presents an D overview of 48 DPB cases from other regions of the world including the US, European countries and Australia. Identification of DPB cases from different racial groups across the globe indicates toward a need to educate pulmonologists to correctly diagnose and initiate treatment. Keywords Diffuse panbronchiolitis (DPB) is a rare inflammatory lung disease. It was first identified in 1969 and is Diffuse panbronchiolitis, erythromycin, interstitial well recognized in East Asian countries such as Japan, China, Taiwan, and Korea.1 ‘Diffuse’ and ‘pan’ lung disease, macrolide therapy, rare disease words in the name indicate ‘presence of lesions through both the lungs,’ and inflammation in all layers of bronchioles. Disclosure: Ram Kumar Mishra has nothing to declare in relation to this article. Opinions expressed in this article are the author’s own findings and do At the time of its discovery, DPB had poor prognosis because of recurrent respiratory infections not in any manner reflect or represent the view of the organization to which he is affiliated. leading to respiratory failure. In the years following the initial description of DPB in Japan, cases were Compliance with Ethics: This study involves a review of also identified in other parts of Asia including China and Taiwan, thus giving it recognition as a distinct the literature and did not involve any studies with human clinical entity. -

Allergic Bronchopulmonary Aspergillosis and Severe Asthma with Fungal Sensitisation
Allergic Bronchopulmonary Aspergillosis and Severe Asthma with Fungal Sensitisation Dr Rohit Bazaz National Aspergillosis Centre, UK Manchester University NHS Foundation Trust/University of Manchester ~ ABPA -a41'1 Severe asthma wl'th funga I Siens itisat i on Subacute IA Chronic pulmonary aspergillosjs Simp 1Ie a:spe rgmoma As r§i · bronchitis I ram une dysfu net Ion Lun· damage Immu11e hypce ractivitv Figure 1 In t@rarctfo n of Aspergillus Vliith host. ABP A, aHerg tc broncho pu~ mo na my as µe rgi ~fos lis; IA, i nvas we as ?@rgiH os 5. MANCHl·.'>I ER J:-\2 I Kosmidis, Denning . Thorax 2015;70:270–277. doi:10.1136/thoraxjnl-2014-206291 Allergic Fungal Airway Disease Phenotypes I[ Asthma AAFS SAFS ABPA-S AAFS-asthma associated with fu ngaIsensitization SAFS-severe asthma with funga l sensitization ABPA-S-seropositive a llergic bronchopulmonary aspergi ll osis AB PA-CB-all ergic bronchopulmonary aspergi ll osis with central bronchiectasis Agarwal R, CurrAlfergy Asthma Rep 2011;11:403 Woolnough K et a l, Curr Opin Pulm Med 2015;21:39 9 Stanford Lucile Packard ~ Children's. Health Children's. Hospital CJ Scanford l MEDICINE Stanford MANCHl·.'>I ER J:-\2 I Aspergi 11 us Sensitisation • Skin testing/specific lgE • Surface hydroph,obins - RodA • 30% of patients with asthma • 13% p.atients with COPD • 65% patients with CF MANCHl·.'>I ER J:-\2 I Alternar1a• ABPA •· .ABPA is an exagg·erated response ofthe imm1une system1 to AspergUlus • Com1pUcatio n of asthm1a and cystic f ibrosis (rarell·y TH2 driven COPD o r no identif ied p1 rior resp1 iratory d isease) • ABPA as a comp1 Ucation of asth ma affects around 2.5% of adullts. -

Vocal Cord Dysfunction: Analysis of 27 Cases and Updated Review of Pathophysiology & Management
THIEME Original Research 125 Vocal Cord Dysfunction: Analysis of 27 Cases and Updated Review of Pathophysiology & Management Shibu George1 Sandeep Suresh2 1 Department of ENT, Government Medical College, Kottayam, Kerala, India Address for correspondence ShibuGeorge,MS,DNB,Charivukalayil 2 Department of ENT, Little Flower Hospital, Ernakulam, Kerala, India (House), Ettumanoor, Kottayam 686631, Kerala, India (e-mail: [email protected]; [email protected]). Int Arch Otorhinolaryngol 2019;23:125–130. Abstract Introduction Vocal cord dysfunction is characterized by unintentional paradoxical vocal cord movement resulting in abnormal inappropriate adduction, especially during inspiration; this predominantly manifests as unresponsive asthma or unexplained stridor. It is prudent to be well informed about the condition, since the primary presentation may mask other airway disorders. Objective This descriptive study was intended to analyze presentations of vocal cord dysfunction in a tertiary care referral hospital. The current understanding regarding the pathophysiology and management of the condition were also explored. Methods A total of 27 patients diagnosed with vocal cord dysfunction were analyzed based on demographic characteristics, presentations, associations and examination findings. The mechanism of causation, etiological factors implicated, diagnostic considerations and treatment options were evaluated by analysis of the current literature. Results Therewasastrongfemalepredilection noted among the study population (n ¼ 27), which had a mean age of 31. The most common presentations were stridor Keywords (44%) and refractory asthma (41%). Laryngopharyngeal reflux disease was the most ► Vocal Cord common association in the majority (66%) of the patients, with a strong overlay of Dysfunction anxiety, demonstrable in 48% of the patients. ► paradoxical vocal Conclusion Being aware of the condition is key to avoid misdiagnosis in vocal cord cord motion dysfunction. -

Diffuse Panbronchiolitis
C M Chu et al • Diffuse Panbronchiolitis DIFFUSE PANBRONCHIOLITIS : A MIMICKER OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE Dr. Chung-ming Chu, MBBS(HK) Dr. Man-fuk Leung, MBBS(HK) FHKCP FHKAM FHKCP FHKAM(Medicine) (Medicine), FRCP(Edin) Senior Medical Officer Consultant and Chief of Service Dr. Cho-yiu Yung, MBBS(HK) FHKCP FHKAM Department of Medicine and Geriatrics (Medicine) United Christian Hospital, Senior Medical Officer 130 Hip Wo Street, Kwun Tong, Dr. Wah-shing Leung, MBChB(CUHK) MRCP(UK) Kowloon, Hong Kong Medical Officer J HK Geriatr Soc 1999;9:29 - 32 Received in revised form on 29 June 1998 Address correspondence to: Dr. CY Yung Summary on level ground and he was home bound, requiring We report a 69-year-old patient presenting with assistance in his activities of daily living. Positive chronic productive cough, progressive dyspnoea, physical findings included diffuse wheezes and airflow obstruction and respiratory failure. He was crackles over the chest. He sought treatment in mislabeled as having chronic obstructive pulmonary various places before seeing us and had been disease (COPD) in the past. Review of his clinical labeled as having COPD, chronic bronchitis and and radiological features finally led to a diagnosis congestive heart failure in the past, and treated as of diffuse panbronchiolitis (DPB). He responded such without improvement. dramatically to long-term low dose erythromycin. The Initial investigations showed a normal blood clinical, radiological and pathological features of the picture and differential counts, biochemistry, renal condition are reviewed. It is important not to miss and liver profiles. Chest radiographs showed diffuse the diagnosis of DPB as it is a potentially treatable micronodules of 2 to 3 mm diameter mainly located condition. -

Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19
University of Kentucky UKnowledge Pharmaceutical Sciences Faculty Publications Pharmaceutical Sciences 2-12-2021 Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19 Vincent J. Venditto University of Kentucky, [email protected] Dalia Haydar St. Jude Children’s Research Hospital Ahmed K. Abdel-Latif University of Kentucky, [email protected] John C. Gensel University of Kentucky, [email protected] See next page for additional authors Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Follow this and additional works at: https://uknowledge.uky.edu/ps_facpub Part of the Medical Immunology Commons, and the Pharmacy and Pharmaceutical Sciences Commons Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19 Digital Object Identifier (DOI) https://doi.org/10.3389/fimmu.2021.574425 Notes/Citation Information Published in Frontiers in Immunology, v. 12, article 574425. © 2021 Venditto, Haydar, Abdel-Latif, Gensel, Anstead, Pitts, Creameans, Kopper, Peng and Feola This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. Authors Vincent J. Venditto, Dalia Haydar, Ahmed K. Abdel-Latif, John C. Gensel, Michael I. Anstead, Michelle G. Pitts, Jarrod W. Creameans, Timothy J. Kopper, Chi Peng, and David J. -

To Honeycombing in Idiopathic Pulmonary Fibrosis
Piciucchi et al. BMC Pulmonary Medicine (2016) 16:87 DOI 10.1186/s12890-016-0245-x CORRESPONDENCE Open Access From “traction bronchiectasis” to honeycombing in idiopathic pulmonary fibrosis: A spectrum of bronchiolar remodeling also in radiology? Sara Piciucchi1*, Sara Tomassetti2, Claudia Ravaglia2, Christian Gurioli2, Carlo Gurioli2, Alessandra Dubini3, Angelo Carloni4, Marco Chilosi5, Thomas V Colby6 and Venerino Poletti2,7 Abstract Background: The diagnostic and prognostic impact of traction bronchiectasis on high resolution CT scan (HRCT) in patients suspected to have idiopathic pulmonary fibrosis (IPF) is increasing significantly. Main body: Recent data demonstrated that cysts in honeycombing areas are covered by epithelium expressing bronchiolar markers. In IPF bronchiolization is the final consequence of a variety of pathogenic events starting from alveolar stem cell exhaustion, and ending in a abnormal/dysplastic proliferation of bronchiolar epithelium. CT scan features of traction bronchiectasis and honeycombing should be interpreted under the light of these new pathogenetic and morphologic considerations. Short conclusion: We suggest that in IPF subjects traction bronchiectasis and honeycombing -now defined as distinct entities on HRCT scan- are actually diverse aspects of a continuous spectrum of lung remodeling. Keywords: Traction bronchiectasis, Honeycombing, Fibroblastic Foci, Bronchiolar dysplastic proliferation Background Mechanical stress may contribute to the subpleural Histologically, Usual Interstitial Pneumonia (UIP) is and usually basilar localization of UIP changes [10, 11]. characterized by a combination of “patchy fibrosis” and The final stage of this “bronchiolization” process cor- fibroblastic foci displaying a “patchwork pattern”. Disease responds radiologically to honeycombing, typically seen progression is characterized by the appearance of air- first in the subpleural regions of the lower lobes [2, 3]. -

Middle Airway Obstructiondit May Be Happening Under Our Noses Philip G Bardin,1 Sebastian L Johnston,2 Garun Hamilton1
Thorax Online First, published on July 19, 2012 as 10.1136/thoraxjnl-2012-202221 Chest clinic OPINION Thorax: first published as 10.1136/thoraxjnl-2012-202221 on 19 July 2012. Downloaded from Middle airway obstructiondit may be happening under our noses Philip G Bardin,1 Sebastian L Johnston,2 Garun Hamilton1 < Additional materials are ABSTRACT for this conceptual error to be refuted.2 The virus is published online only. To view Background Lower airway obstruction has evolved to now understood to spread from nose to lung, and these files please visit the denote pathologies associated with diseases of the lung, appropriate recognition of the close association journal online (http://dx.doi.org/ 10.1136/ whereas, conditions proximal to the lung embody upper between upper and lower airway pathologies has thoraxjnl-2012-202221/content/ airway obstruction. This approach has disconnected had positive outcomes. An example is novel strat- early/recent). diseases of the larynx and trachea from the lung, and egies that may not be able to prevent colds, but 1Lung and Sleep Medicine, removed the ‘middle airway’ from the interest and that could ameliorate virus asthma exacerbations Monash University and Hospital involvement of respiratory physicians and scientists. through the use of inhaled interferon.3 and Monash Institute of Medical However, recent studies have indicated that dysfunction Accumulating evidence suggests that the middle Research (MIMR), Melbourne, of this anatomical region may be a key component of Australia airway plays a key role in airway obstruction either 2Respiratory Medicine, Imperial overall airway obstruction, either independently or in independently or with coexisting lung disease. -
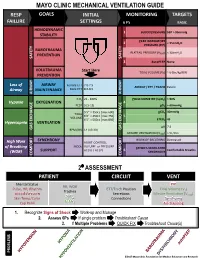
Mechanical Ventilation Guide
MAYO CLINIC MECHANICAL VENTILATION GUIDE RESP GOALS INITIAL MONITORING TARGETS FAILURE SETTINGS 6 P’s BASIC HEMODYNAMIC 1 BLOOD PRESSURE SBP > 90mmHg STABILITY PEAK INSPIRATORY 2 < 35cmH O PRESSURE (PIP) 2 BAROTRAUMA PLATEAU PRESSURE (P ) < 30cmH O PREVENTION PLAT 2 SAFETY SAFETY 3 AutoPEEP None VOLUTRAUMA Start Here TIDAL VOLUME (V ) ~ 6-8cc/kg IBW PREVENTION T Loss of AIRWAY Female ETT 7.0-7.5 AIRWAY / ETT / TRACH Patent Airway MAINTENANCE Male ETT 8.0-8.5 AIRWAY AIRWAY FiO2 21 - 100% PULSE OXIMETRY (SpO2) > 90% Hypoxia OXYGENATION 4 PEEP 5 [5-15] pO2 > 60mmHg 5’5” = 350cc [max 600] pCO2 40mmHg TIDAL 6’0” = 450cc [max 750] 5 VOLUME 6’5” = 500cc [max 850] ETCO2 45 Hypercapnia VENTILATION pH 7.4 GAS GAS EXCHANGE BPM (RR) 14 [10-30] GAS EXCHANGE MINUTE VENTILATION (VMIN) > 5L/min SYNCHRONY WORK OF BREATHING Decreased High Work ASSIST CONTROL MODE VOLUME or PRESSURE of Breathing PATIENT-VENTILATOR AC (V) / AC (P) 6 Comfortable Breaths (WOB) SUPPORT SYNCHRONY COMFORT COMFORT 2⁰ ASSESSMENT PATIENT CIRCUIT VENT Mental Status PIP RR, WOB Pulse, HR, Rhythm ETT/Trach Position Tidal Volume (V ) Trachea T Blood Pressure Secretions Minute Ventilation (V ) SpO MIN Skin Temp/Color 2 Connections Synchrony ETCO Cap Refill 2 Air-Trapping 1. Recognize Signs of Shock Work-up and Manage 2. Assess 6Ps If single problem Troubleshoot Cause 3. If Multiple Problems QUICK FIX Troubleshoot Cause(s) PROBLEMS ©2017 Mayo Clinic Foundation for Medical Education and Research CAUSES QUICK FIX MANAGEMENT Bleeding Hemostasis, Transfuse, Treat cause, Temperature control HYPOVOLEMIA Dehydration Fluid Resuscitation (End points = hypoxia, ↑StO2, ↓PVI) 3rd Spacing Treat cause, Beware of hypoxia (3rd spacing in lungs) Pneumothorax Needle D, Chest tube Abdominal Compartment Syndrome FLUID Treat Cause, Paralyze, Surgery (Open Abdomen) OBSTRUCTED BLOOD RETURN Air-Trapping (AutoPEEP) (if not hypoxic) Pop off vent & SEE SEPARATE CHART PEEP Reduce PEEP Cardiac Tamponade Pericardiocentesis, Drain. -

Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders
88 XAOIOOIOO Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders Yong Whee Bahk, M.D. and Soo Kyo Chung, M.D. Introduction As a coordinated research project of the International Atomic Energy Agency (IAEA) a multicentre joint study on radioaerosol lung scan using the BARC nebulizer [1] has prospectively been carried out during 1988-1992 with the participation of 10 member countries in Asia [Bangladesh, China, India, Indonesia, Japan, Korea, Pakistan, Philippines, Singapore and Thailand]. The study was designed so that it would primarily cover chronic obstructive pulmonary disease (COPD) and the other related and common pulmonary diseases. The study also included normal controls and asymptomatic smokers. The purposes of this presentation are three fold: firstly, to document the useful- ness of the nebulizer and the validity of user's protocol in imaging COPD and other lung diseases; secondly, to discuss scan features of the individual COPD and other disorders studied and thirdly, to correlate scan alterations with radiographie find- ings. Before proceeding with a systematic analysis of aerosol scan patterns in the disease groups, we documented normal pattern. The next step was the assessment of scan features in those who had been smoking for more than several years but had no symptoms or signs referable to airways. The lung diseases we analyzed included COPD [emphysema, chronic bronchitis, asthma and bronchiectasis], bron- chial obstruction, compensatory overinflation and other common lung diseases such as lobar pneumonia, tuberculosis, interstitial fibrosis, diffuse panbronchiolitis, lung edema and primary and metastatic lung cancers. Lung embolism, inhalation burns and glue-sniffer's lung are seperately discussed by Dr. -

Allergic Bronchopulmonary Aspergillosis: a Perplexing Clinical Entity Ashok Shah,1* Chandramani Panjabi2
Review Allergy Asthma Immunol Res. 2016 July;8(4):282-297. http://dx.doi.org/10.4168/aair.2016.8.4.282 pISSN 2092-7355 • eISSN 2092-7363 Allergic Bronchopulmonary Aspergillosis: A Perplexing Clinical Entity Ashok Shah,1* Chandramani Panjabi2 1Department of Pulmonary Medicine, Vallabhbhai Patel Chest Institute, University of Delhi, Delhi, India 2Department of Respiratory Medicine, Mata Chanan Devi Hospital, New Delhi, India This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. In susceptible individuals, inhalation of Aspergillus spores can affect the respiratory tract in many ways. These spores get trapped in the viscid spu- tum of asthmatic subjects which triggers a cascade of inflammatory reactions that can result in Aspergillus-induced asthma, allergic bronchopulmo- nary aspergillosis (ABPA), and allergic Aspergillus sinusitis (AAS). An immunologically mediated disease, ABPA, occurs predominantly in patients with asthma and cystic fibrosis (CF). A set of criteria, which is still evolving, is required for diagnosis. Imaging plays a compelling role in the diagno- sis and monitoring of the disease. Demonstration of central bronchiectasis with normal tapering bronchi is still considered pathognomonic in pa- tients without CF. Elevated serum IgE levels and Aspergillus-specific IgE and/or IgG are also vital for the diagnosis. Mucoid impaction occurring in the paranasal sinuses results in AAS, which also requires a set of diagnostic criteria. Demonstration of fungal elements in sinus material is the hall- mark of AAS. -

A Case of Idiopathic Pulmonary Fibrosis Treated with Clarithromycin
CASE REPORT East J Med 21(1): 39-41, 2016 A case of idiopathic pulmonary fibrosis treated with clarithromycin Masashi Ohe1,* and Satoshi Hashino2 1Department of General Medicine, Hokkaido Social Insurance Hospital, Sapporo, Japan 2Hokkaido University, Health Care Center, Sapporo, Japan ABSTRACT We report a case of idiopathic pulmonary fibrosis (IPF) treated successfully using clarithromycin (CAM). A 64-year-old male patient suffering from IPF which had been diagnosed at 59 years old, presented with slowly progressive dyspnea. Until this time, his condition had been almost stable. He was diagnosed with exacerbation of IPF, based on exacerbation of lung ausculation, O2 saturation by pulse oxymetry, Krebs von den Lungen 6 levels and chest computed tomography findings of reticular opacities. He was successfully treated using CAM in expectation of its anti-inflammatory effects. This case shows that treatment using CAM may be effective in some cases of IPF. Key Words: Idiopathic pulmonary fibrosis, Clarithromycin Introduction protein (CRP), lactate dehydrogenase (LDH), and Krebs von den Lungen (KL)-6 levels which are Idiopathic pulmonary fibrosis (IPF) is progressive known to indicate the activity of interstitial and fatal lung disease. Recent study suggested pneumonia (4), and CT findings remained almost pirfenidone might be effective in slowing the unchanged, he did not receive any treatment. Six decline of lung function on early-stage IPF months before this episode of dyspnea, SpO2 persons (1). However, despite multiple recent indicated 94% in room air at rest. CRP, LDH, and clinical trials, no definite therapy other than lung KL-6 levels were 0.2 mg/dL (normal range <0.3 transplantation is known to alter survival (2). -

Klebsiella Pneumoniae in Diffuse Panbronchiolitis
※ 附來的圖四解析度和原來一樣哦 , 應該不是原始檔案 , 如沒有原圖印刷就是字不漂亮 DOI:10.6314/JIMT.202012_31(6).07 內科學誌 2020:31:432-436 Klebsiella Pneumoniae in Diffuse Panbronchiolitis Hwee-Kheng Lim1, Sho-Ting Hung2, and Shih-Yi Lee3,4 1Division of Infectious Diseases, Department of Medicine, 2Department of Radiology, 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Taitung MacKay Memorial Hospital, Taitung, Taiwan; 4MacKay Medicine, Nursing & Management College Abstract Diffuse panbronchiolitis (DPB) is a chronic, idiopathic, rare but lethal inflammatory airway disease resulting in distal airway dilation, obstructive lung disease, hypoxemia, and increased in the susceptibility of bacteria colonization. The case reports that a patient with destructive lung gets pneumonia by Klebsiella pneu- moniae cured by bactericidal antibiotics, but K. pneumoniae in the airway is eradicated with erythromycin, which therefore highlights not only the importance to the testing for DPB in suspicious patients, but also the therapeutic strategies in managing DPB with K. pneumoniae in the airways. (J Intern Med Taiwan 2020; 31: 432-436) Key Words: Klebsiella pneumoniae, Panbronchiolitis Introduction the survival of the patients with DPB in the literature also reduces P. aeruginosa isolated from the sputum. Diffuse panbronchiolitis (DPB) is a chronic P. aeruginosa in sputum appears to accelerate the inflammatory disease of the airways, and the diag- destructive process3. However, the role of Klebsiella nosis depends on the clinical symptoms, physical pneumoniae in DPB has never been reported. We signs, typical chest radiographic findings, low FEV1 present a patient with DPB and K. pneumoniae iso- (< 70%) in pulmonary function tests, low arterial lated from sputum, refractory to ampicillin/sulbac- partial pressure (< 80 mmHg), elevated cold hem- tam, but eradicated by erythromycin.