Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diffuse Panbronchiolitis Is Not Restricted to East Asia—A Mini Literature Review
Review Interstitial Lung Disease Diffuse Panbronchiolitis is not Restricted to East Asia—a Mini Literature Review Ram Kumar Mishra Epidemiology and HEOR Team, ODC 3, Tata Consultancy Services, Thane (W), Maharashtra, India DOI: https://doi.org/10.17925/USRPD.2017.12.02.30 iffuse panbronchiolitis (DPB) is a rare inflammatory lung disease, and is well recognized in East Asian countries like Japan, China, Taiwan and Korea. Over the years, sporadic DPB cases have been reported worldwide from other countries. This literature review presents an D overview of 48 DPB cases from other regions of the world including the US, European countries and Australia. Identification of DPB cases from different racial groups across the globe indicates toward a need to educate pulmonologists to correctly diagnose and initiate treatment. Keywords Diffuse panbronchiolitis (DPB) is a rare inflammatory lung disease. It was first identified in 1969 and is Diffuse panbronchiolitis, erythromycin, interstitial well recognized in East Asian countries such as Japan, China, Taiwan, and Korea.1 ‘Diffuse’ and ‘pan’ lung disease, macrolide therapy, rare disease words in the name indicate ‘presence of lesions through both the lungs,’ and inflammation in all layers of bronchioles. Disclosure: Ram Kumar Mishra has nothing to declare in relation to this article. Opinions expressed in this article are the author’s own findings and do At the time of its discovery, DPB had poor prognosis because of recurrent respiratory infections not in any manner reflect or represent the view of the organization to which he is affiliated. leading to respiratory failure. In the years following the initial description of DPB in Japan, cases were Compliance with Ethics: This study involves a review of also identified in other parts of Asia including China and Taiwan, thus giving it recognition as a distinct the literature and did not involve any studies with human clinical entity. -

Diffuse Panbronchiolitis
C M Chu et al • Diffuse Panbronchiolitis DIFFUSE PANBRONCHIOLITIS : A MIMICKER OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE Dr. Chung-ming Chu, MBBS(HK) Dr. Man-fuk Leung, MBBS(HK) FHKCP FHKAM FHKCP FHKAM(Medicine) (Medicine), FRCP(Edin) Senior Medical Officer Consultant and Chief of Service Dr. Cho-yiu Yung, MBBS(HK) FHKCP FHKAM Department of Medicine and Geriatrics (Medicine) United Christian Hospital, Senior Medical Officer 130 Hip Wo Street, Kwun Tong, Dr. Wah-shing Leung, MBChB(CUHK) MRCP(UK) Kowloon, Hong Kong Medical Officer J HK Geriatr Soc 1999;9:29 - 32 Received in revised form on 29 June 1998 Address correspondence to: Dr. CY Yung Summary on level ground and he was home bound, requiring We report a 69-year-old patient presenting with assistance in his activities of daily living. Positive chronic productive cough, progressive dyspnoea, physical findings included diffuse wheezes and airflow obstruction and respiratory failure. He was crackles over the chest. He sought treatment in mislabeled as having chronic obstructive pulmonary various places before seeing us and had been disease (COPD) in the past. Review of his clinical labeled as having COPD, chronic bronchitis and and radiological features finally led to a diagnosis congestive heart failure in the past, and treated as of diffuse panbronchiolitis (DPB). He responded such without improvement. dramatically to long-term low dose erythromycin. The Initial investigations showed a normal blood clinical, radiological and pathological features of the picture and differential counts, biochemistry, renal condition are reviewed. It is important not to miss and liver profiles. Chest radiographs showed diffuse the diagnosis of DPB as it is a potentially treatable micronodules of 2 to 3 mm diameter mainly located condition. -

Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders
88 XAOIOOIOO Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders Yong Whee Bahk, M.D. and Soo Kyo Chung, M.D. Introduction As a coordinated research project of the International Atomic Energy Agency (IAEA) a multicentre joint study on radioaerosol lung scan using the BARC nebulizer [1] has prospectively been carried out during 1988-1992 with the participation of 10 member countries in Asia [Bangladesh, China, India, Indonesia, Japan, Korea, Pakistan, Philippines, Singapore and Thailand]. The study was designed so that it would primarily cover chronic obstructive pulmonary disease (COPD) and the other related and common pulmonary diseases. The study also included normal controls and asymptomatic smokers. The purposes of this presentation are three fold: firstly, to document the useful- ness of the nebulizer and the validity of user's protocol in imaging COPD and other lung diseases; secondly, to discuss scan features of the individual COPD and other disorders studied and thirdly, to correlate scan alterations with radiographie find- ings. Before proceeding with a systematic analysis of aerosol scan patterns in the disease groups, we documented normal pattern. The next step was the assessment of scan features in those who had been smoking for more than several years but had no symptoms or signs referable to airways. The lung diseases we analyzed included COPD [emphysema, chronic bronchitis, asthma and bronchiectasis], bron- chial obstruction, compensatory overinflation and other common lung diseases such as lobar pneumonia, tuberculosis, interstitial fibrosis, diffuse panbronchiolitis, lung edema and primary and metastatic lung cancers. Lung embolism, inhalation burns and glue-sniffer's lung are seperately discussed by Dr. -

A Case of Idiopathic Pulmonary Fibrosis Treated with Clarithromycin
CASE REPORT East J Med 21(1): 39-41, 2016 A case of idiopathic pulmonary fibrosis treated with clarithromycin Masashi Ohe1,* and Satoshi Hashino2 1Department of General Medicine, Hokkaido Social Insurance Hospital, Sapporo, Japan 2Hokkaido University, Health Care Center, Sapporo, Japan ABSTRACT We report a case of idiopathic pulmonary fibrosis (IPF) treated successfully using clarithromycin (CAM). A 64-year-old male patient suffering from IPF which had been diagnosed at 59 years old, presented with slowly progressive dyspnea. Until this time, his condition had been almost stable. He was diagnosed with exacerbation of IPF, based on exacerbation of lung ausculation, O2 saturation by pulse oxymetry, Krebs von den Lungen 6 levels and chest computed tomography findings of reticular opacities. He was successfully treated using CAM in expectation of its anti-inflammatory effects. This case shows that treatment using CAM may be effective in some cases of IPF. Key Words: Idiopathic pulmonary fibrosis, Clarithromycin Introduction protein (CRP), lactate dehydrogenase (LDH), and Krebs von den Lungen (KL)-6 levels which are Idiopathic pulmonary fibrosis (IPF) is progressive known to indicate the activity of interstitial and fatal lung disease. Recent study suggested pneumonia (4), and CT findings remained almost pirfenidone might be effective in slowing the unchanged, he did not receive any treatment. Six decline of lung function on early-stage IPF months before this episode of dyspnea, SpO2 persons (1). However, despite multiple recent indicated 94% in room air at rest. CRP, LDH, and clinical trials, no definite therapy other than lung KL-6 levels were 0.2 mg/dL (normal range <0.3 transplantation is known to alter survival (2). -

Klebsiella Pneumoniae in Diffuse Panbronchiolitis
※ 附來的圖四解析度和原來一樣哦 , 應該不是原始檔案 , 如沒有原圖印刷就是字不漂亮 DOI:10.6314/JIMT.202012_31(6).07 內科學誌 2020:31:432-436 Klebsiella Pneumoniae in Diffuse Panbronchiolitis Hwee-Kheng Lim1, Sho-Ting Hung2, and Shih-Yi Lee3,4 1Division of Infectious Diseases, Department of Medicine, 2Department of Radiology, 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Taitung MacKay Memorial Hospital, Taitung, Taiwan; 4MacKay Medicine, Nursing & Management College Abstract Diffuse panbronchiolitis (DPB) is a chronic, idiopathic, rare but lethal inflammatory airway disease resulting in distal airway dilation, obstructive lung disease, hypoxemia, and increased in the susceptibility of bacteria colonization. The case reports that a patient with destructive lung gets pneumonia by Klebsiella pneu- moniae cured by bactericidal antibiotics, but K. pneumoniae in the airway is eradicated with erythromycin, which therefore highlights not only the importance to the testing for DPB in suspicious patients, but also the therapeutic strategies in managing DPB with K. pneumoniae in the airways. (J Intern Med Taiwan 2020; 31: 432-436) Key Words: Klebsiella pneumoniae, Panbronchiolitis Introduction the survival of the patients with DPB in the literature also reduces P. aeruginosa isolated from the sputum. Diffuse panbronchiolitis (DPB) is a chronic P. aeruginosa in sputum appears to accelerate the inflammatory disease of the airways, and the diag- destructive process3. However, the role of Klebsiella nosis depends on the clinical symptoms, physical pneumoniae in DPB has never been reported. We signs, typical chest radiographic findings, low FEV1 present a patient with DPB and K. pneumoniae iso- (< 70%) in pulmonary function tests, low arterial lated from sputum, refractory to ampicillin/sulbac- partial pressure (< 80 mmHg), elevated cold hem- tam, but eradicated by erythromycin. -

Therapeutic Efficacy of Macrolides in Management of Patients with Mild
www.nature.com/scientificreports OPEN Therapeutic efcacy of macrolides in management of patients with mild COVID‑19 Alaa Rashad1, Asmaa Nafady2,3, Mohammed H. Hassan4*, Haggagy Mansour1, Usama Taya5, Shamardan Ezzeldin S. Bazeed6, Zaki F. Aref5, Mennatallah Ali Abdelrhman Sayed7, Hanaa Nafady‑Hego8 & Aida A. Abdelmaksoud5 Evidence on the efcacy of adding macrolides (azithromycin or clarithromycin) to the treatment regimen for COVID‑19 is limited. We testify whether adding azithromycin or clarithromycin to a standard of care regimen was superior to standard of supportive care alone in patients with mild COVID‑19.This randomized trial included three groups of patients with COVID‑19. The azithromycin group included, 107 patients who received azithromycin 500 mg/24 h for 7 days, the clarithromycin group included 99 patients who received clarithromycin 500 /12 h for 7 days, and the control group included 99 patients who received standard care only. All three groups received only symptomatic treatment for control of fever and cough .Clinical and biochemical evaluations of the study participants including assessment of the symptoms duration, real‑time reverse transcription‑ polymerase chain reaction (rRT‑PCR), C‑reactive protein (CRP), serum ferritin, D‑dimer, complete blood count (CBC), in addition to non‑contrast chest computed tomography (CT), were performed. The overall results revealed signifcant early improvement of symptoms (fever, dyspnea and cough) in patients treated with either azithromycin or clarithromycin compared to control group, also there was signifcant early conversion of SARS‑CoV‑2 PCR to negative in patients treated with either azithromycin or clarithromycin compared to control group (p < 0.05 for all).There was no signifcant diference in time to improvement of fever, cough, dyspnea, anosmia, gastrointestinal tract "GIT" symptoms and time to PCR negative conversion between patients treated with azithromycin compared to patients treated with clarithromycin (p > 0.05 for all). -

Long-Term Efficacy of Macrolide Treatment in Idiopathic Pulmonary Fibrosis: a Retrospective Analysis
Original article: Clinical research SARCOIDOSIS VASCULITIS AND DIFFUSE LUNG DISEASES 2016; 33; 242-246 © Mattioli 1885 Long-term efficacy of macrolide treatment in idiopathic pulmonary fibrosis: a retrospective analysis Naoyuki Kuse, Shinji Abe, Hiroki Hayashi, Koichiro Kamio, Yoshinobu Saito, Jiro Usuki, Arata Azuma, Shoji Kudoh, Akihiko Gemma Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan Abstract. Background and objective: There is growing evidence for anti-inflammatory activities of macrolides in chronic respiratory diseases, such as diffuse panbronchiolitis, cystic fibrosis, or chronic bronchitis. The long- term effect of macrolides in idiopathic pulmonary fibrosis (IPF) is unknown. This study was aimed to investigate the effect of macrolide therapy on the frequency of acute exacerbation (AE) and the mortality in IPF. Methods: A total 52 IPF patients who were treated by combination of conventional agents with or without macrolides were retrospectively reviewed. The primary endpoint was the incidence of AE in IPF patients. We also observed survival rate after the treatment with or without macrolides. Results: AE was observed in 4 of 29 cases (13.8%) treated with macrolides and 8 of 23 cases (34.8%) treated without macrolides, respectively during 36 months. AE free survival rate of macrolide group was significantly better than that of non-macrolide group (logrank p=0.027). Survival rate of IPF patients with macrolide therapy was significantly better than that of patients without macrolide therapy (p=0.047). Conclusion: Our results indicate the potential beneficial efficacy of macro- lide therapy combined with oral corticosteroids, immunosuppressive or anti-fibrotic agents in IPF. -

Macrolide Therapy in Cryptogenic Organizing Pneumonia: a Case Report and Literature Review
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 829-834, 2015 Macrolide therapy in cryptogenic organizing pneumonia: A case report and literature review QUN‑LI DING, DAN LV, BI‑JIONG WANG, QIAO‑LI ZHANG, YI‑MING YU, SHI‑FANG SUN, ZHONG-BO CHEN, HONG-YING MA and ZAI-CHUN DENG Department of Respiratory Medicine, Affiliated Hospital, School of Medicine, Ningbo University, Ningbo, Zhejiang 315020, P.R. China Received May 9, 2014; Accepted December 15, 2014 DOI: 10.3892/etm.2015.2183 Abstract. Cryptogenic organizing pneumonia (COP) is a pneumonia (1,2). Secondary OP is associated with a number of pulmonary disorder associated with nonspecific clinical entities, including drugs, infections, malignancies, connective presentations. The macrolide class of antimicrobial agents is tissue diseases, organ transplantation, radiotherapy and the widely used to treat infectious and inflammatory respiratory inhalation of harmful gases. COP mainly involves the alveoli, diseases in humans. The present study reports a case of COP alveolar ducts and small airways; however, the lung intersti- that was effectively treated with azithromycin in combination tium may also be involved. It is considered as an inflammatory with glucocorticoid. A literature review of similar cases is disease and diagnosed based on the clinical, radiographic and also presented. It was found that all COP patients in the litera- pathological findings following the exclusion of diseases asso- ture received macrolide treatment, including six cases with ciated with secondary OP (3). Glucocorticoids are effective in unknown clinical outcomes. For the remaining 29 patients, the treatment of COP. However, glucocorticoids usually take a 20 patients initially received the macrolide as a single therapy longer time to take effect, and this results in severe side-effects. -

Legionella Spp. in Acute Exacerbations of Chronic Obstructive Pulmonary Disease: What Is the Evidence?
Copyright #ERS Journals Ltd 2002 Eur Respir J 2002; 19: 387–389 European Respiratory Journal DOI: 10.1183/09031936.02.00281402 ISSN 0903-1936 Printed in UK – all rights reserved EDITORIAL Legionella spp. in acute exacerbations of chronic obstructive pulmonary disease: what is the evidence? S. Ewig Acute exacerbations are a frequent complication pathogens of acute exacerbations of COPD and during the clinical course of chronic obstructive should antimicrobial treatment regimens, targeted pulmonary disease (COPD). A recent monograph against these pathogens be designed? dealing with COPD exacerbations demonstrated Up to now, Legionella spp. have not been reported that virtually all issues related to the management of to form part of the microbial patterns of acute acute exacerbations remain unsettled and contro- exacerbations. This may simply reflect the principal versial, including the definition, aetiology, microbial methodological problems of diagnosing such infec- patterns, and antimicrobial treatment of this con- tions. Legionella spp. can only rarely be cultured from dition [1]. This is of particular concern in view of the sputum, and bronchoalveolar lavage fluid is usually high burden of this complication on public health not suitable in COPD patients with acute exacer- resources. bations. In fact, performing bronchoalveolar may With regards to microbial patterns and their pos- prove harmful in these patients. Antigen detection, sible involvement in the aetiology of acute exacer- although highly specific and sensitive, exclusively bations, it is a common view that Haemophilus covers infections by Legionella pneumophila sero- influenzae, Streptococcus pneumoniae, and Moraxella group 1. A paired serum for serology is only rarely catarrhalis are the leading pathogens. -
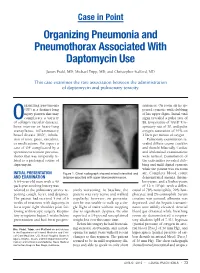
Organizing Pneumonia and Pneumothorax Associated with Daptomycin Use
Case in Point Organizing Pneumonia and Pneumothorax Associated With Daptomycin Use James Prahl, MD; Michael Tripp, MD; and Christopher Stafford, MD This case examines the rare association between the administration of daptomycin and pulmonary toxicity. rganizing pneumonia sentences. On room air he ap- (OP) is a distinct lung peared cyanotic with clubbing injury pattern that may of his upper digits. Initial vital Ocomplicate a variety signs revealed a pulse rate of of collagen vascular diseases, 88, temperature of 100.8º F, re- bone marrow or heart-lung spiratory rate of 30, and pulse transplants, inflammatory oxygen saturation of 91% on bowel disease (IBD), inhala- 2 liters per minute of oxygen. tion of toxic gases, vasculitis, Pulmonary examination re- or medications. We report a vealed diffuse coarse crackles case of OP complicated by a and rhonchi bilaterally. Cardiac spontaneous tension pneumo- and abdominal examinations thorax that was temporally re- were normal. Examination of lated to a prolonged course of the extremities revealed club- daptomycin. bing and mild digital cyanosis while the patient was on room INITIAL PRESENTATION Figure 1. Chest radiograph showed mixed interstitial and air. Complete blood count AND EXAMINATION airspace opacities with upper lobe predominance. demonstrated anemia, throm- A 64-year-old man with a 40 bocytosis, and a leukocytosis pack-year smoking history was of 12 x 103/µL with a differ- referred to the pulmonary service re- sively worsening. At baseline, the ential of 78% neutrophils, 20% lym- porting cough, fever, and dyspnea. patient was very active and walked phocytes, and 2% eosinophils. Serum The patient had received 5 out of 6 regularly; however, on presenta- creatine was normal, albumin was weeks of treatment with daptomycin tion he was unable to walk up a sin- depressed, and the hepatic transami- for a septic right shoulder joint fol- gle flight of stairs without stopping nases were mildly elevated. -

Surgical Pathology of Non-Neoplastic Lung Disease Thomas V
SHORT COURSE Surgical Pathology of Non-Neoplastic Lung Disease Thomas V. Colby, M.D. Department of Laboratory Medicine and Pathology, Mayo Clinic Scottsdale, Scottsdale, Arizona At inception, this course included some neoplastic Case 1: Acute Exacerbation of Idiopathic lesions, but each year, the case mix was changed in Pulmonary Fibrosis response to the comments (and criticism) of the A 51-year-old man had experienced 3 to 4 years attendees. As cases were added and deleted, in the of progressive dyspnea and interstitial infiltrates as end only examples of non-neoplastic lung disease observed on chest radiographs. One week before were discussed. Of the 19 cases used during the death, there was acute worsening of his dyspnea, course of 5 years, the following 8 were selected for with some associated chills and a sore throat. At this presentation. admission, he was hypoxemic, tachypneic, and afe- brile. The clinical impression was an acute (possibly CASES 1, 2, AND 3: IDIOPATHIC INTERSTITIAL infectious) process superimposed on idiopathic PNEUMONIAS AND RELATED LESIONS pulmonary fibrosis (IPF). An open-lung biopsy was performed. The patient died 1 week later. Cultures To the clinician, there are well over 100 causes of and special stains were negative. interstitial pneumonia, but among these, there is a select subgroup that has been called the idiopathic Histologic findings interstitial pneumonias. This group has evolved The open biopsy shows subpleural scarring and from Liebow and Carrington’s (1) classic classifica- microscopic honeycombing, with associated tion of chronic interstitial pneumonias: smooth muscle metaplasia and fibroblastic foci Usual interstitial pneumonia (UIP) (Figs. 1 and 2). -

An Autopsy Case of Diffuse Panbronchiolitis Accompanying Rheumatoid Arthritis
Respiratory Medicine (1996) 90, 175-177 An autopsy case of diffuse panbronchiolitis accompanying rheumatoid arthritis Y. SUGIYAMA*§, K. SAITOH~, S. KANO$ AND S. KITAMURA* Departments of *Pulmonary Medicine and tPathology and SDivision of Clinical Immunology, Jichi Medical School, Tochigi-ken, Japan Introduction morning stiffness of the bilateral knee joints and Diffuse panbronchiolitis is a clinicopathological fingers. At that time, rheumatoid factor was positive entity with the characteristics of chronic recurrent and she had been diagnosed as having classical sinopulmonary infection, respiratory bronchiolitis, rheumatoid arthritis. She was thereafter treated with non-steroidal anti-inflammatory drugs, prednisolone and peribronchiolitis that are diffusely disseminated throughout the bilateral lungs, especially in the lower (5-l 5 mg day - I), D-penicillamine and gold. For the 5 yr preceding her most recent admission, she had lobes (1,2). This disorder is prevalent in Japan but rare in America and Europe. Chronic cough, copious had chronic yellow sputum and cough, and she had been diagnosed with chronic bronchitis. Haemophilus sputum and dyspnoea on exertion are frequent com- plicating symptoms. The radiographic findings con- influenzae had been detected in her sputum 2 yr previously, and, at this admission, Pseudomonas sist of diffusely disseminated fine nodular shadows in the lower lung fields, and hyperinflation of the lungs. aeruginosa was found. Physical examination dis- closed a dyspnoeic and deteriorated woman with Slight bronchiectasis, which appears as tramlines, muscle atrophy and ulnar deviation of the fingers. usually develops in the middle lobe and lingula. In advanced cases, bronchiectasis sometimes extends Course crackles and wheezes could be heard in her bilateral lungs on auscultation.