Assessing Estuary Ecosystem Health: Sampling, Data Analysis and Reporting Protocols
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NPWS Pocket Guide 3E (South Coast)
SOUTH COAST 60 – South Coast Murramurang National Park. Photo: D Finnegan/OEH South Coast – 61 PARK LOCATIONS 142 140 144 WOLLONGONG 147 132 125 133 157 129 NOWRA 146 151 145 136 135 CANBERRA 156 131 148 ACT 128 153 154 134 137 BATEMANS BAY 139 141 COOMA 150 143 159 127 149 130 158 SYDNEY EDEN 113840 126 NORTH 152 Please note: This map should be used as VIC a basic guide and is not guaranteed to be 155 free from error or omission. 62 – South Coast 125 Barren Grounds Nature Reserve 145 Jerrawangala National Park 126 Ben Boyd National Park 146 Jervis Bay National Park 127 Biamanga National Park 147 Macquarie Pass National Park 128 Bimberamala National Park 148 Meroo National Park 129 Bomaderry Creek Regional Park 149 Mimosa Rocks National Park 130 Bournda National Park 150 Montague Island Nature Reserve 131 Budawang National Park 151 Morton National Park 132 Budderoo National Park 152 Mount Imlay National Park 133 Cambewarra Range Nature Reserve 153 Murramarang Aboriginal Area 134 Clyde River National Park 154 Murramarang National Park 135 Conjola National Park 155 Nadgee Nature Reserve 136 Corramy Regional Park 156 Narrawallee Creek Nature Reserve 137 Cullendulla Creek Nature Reserve 157 Seven Mile Beach National Park 138 Davidson Whaling Station Historic Site 158 South East Forests National Park 139 Deua National Park 159 Wadbilliga National Park 140 Dharawal National Park 141 Eurobodalla National Park 142 Garawarra State Conservation Area 143 Gulaga National Park 144 Illawarra Escarpment State Conservation Area Murramarang National Park. Photo: D Finnegan/OEH South Coast – 63 BARREN GROUNDS BIAMANGA NATIONAL PARK NATURE RESERVE 13,692ha 2,090ha Mumbulla Mountain, at the upper reaches of the Murrah River, is sacred to the Yuin people. -
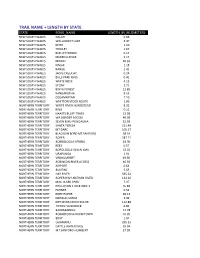
Trail Name + Length by State
TRAIL NAME + LENGTH BY STATE STATE ROAD_NAME LENGTH_IN_KILOMETERS NEW SOUTH WALES GALAH 0.66 NEW SOUTH WALES WALLAGOOT LAKE 3.47 NEW SOUTH WALES KEITH 1.20 NEW SOUTH WALES TROLLEY 1.67 NEW SOUTH WALES RED LETTERBOX 0.17 NEW SOUTH WALES MERRICA RIVER 2.15 NEW SOUTH WALES MIDDLE 40.63 NEW SOUTH WALES NAGHI 1.18 NEW SOUTH WALES RANGE 2.42 NEW SOUTH WALES JACKS CREEK AC 0.24 NEW SOUTH WALES BILLS PARK RING 0.41 NEW SOUTH WALES WHITE ROCK 4.13 NEW SOUTH WALES STONY 2.71 NEW SOUTH WALES BINYA FOREST 12.85 NEW SOUTH WALES KANGARUTHA 8.55 NEW SOUTH WALES OOLAMBEYAN 7.10 NEW SOUTH WALES WHITTON STOCK ROUTE 1.86 NORTHERN TERRITORY WAITE RIVER HOMESTEAD 8.32 NORTHERN TERRITORY KING 0.53 NORTHERN TERRITORY HAASTS BLUFF TRACK 13.98 NORTHERN TERRITORY WA BORDER ACCESS 40.39 NORTHERN TERRITORY SEVEN EMU‐PUNGALINA 52.59 NORTHERN TERRITORY SANTA TERESA 251.49 NORTHERN TERRITORY MT DARE 105.37 NORTHERN TERRITORY BLACKGIN BORE‐MT SANFORD 38.54 NORTHERN TERRITORY ROPER 287.71 NORTHERN TERRITORY BORROLOOLA‐SPRING 63.90 NORTHERN TERRITORY REES 0.57 NORTHERN TERRITORY BOROLOOLA‐SEVEN EMU 32.02 NORTHERN TERRITORY URAPUNGA 1.91 NORTHERN TERRITORY VRDHUMBERT 49.95 NORTHERN TERRITORY ROBINSON RIVER ACCESS 46.92 NORTHERN TERRITORY AIRPORT 0.64 NORTHERN TERRITORY BUNTINE 5.63 NORTHERN TERRITORY HAY RIVER 335.62 NORTHERN TERRITORY ROPER HWY‐NATHAN RIVER 134.20 NORTHERN TERRITORY MAC CLARK PARK 7.97 NORTHERN TERRITORY PHILLIPSON STOCK ROUTE 55.84 NORTHERN TERRITORY FURNER 0.54 NORTHERN TERRITORY PORT ROPER 40.13 NORTHERN TERRITORY NDHALA GORGE 3.49 NORTHERN TERRITORY -

Discovering Bawley Point
Discovering Bawley Point A paradise on the NSW south coast 2 The Bawley Coast Contents Introduction page 3 Our history page 4 Wildlife page 6 Activities pages 8 Shopping page 14 Cafes and restaurants page 15 Vineyards and berry farms page 16 This e-book was written and published by Bill Powell, Bawley Bush Cottages, February 2011. Web: bawleybushcottages.com.au Email: [email protected] 101 Willinga Rd, Bawley Point, NSW 2539, Australia Tel: (61) 2 4457 1580 Bookings: (61) 2 4480 6754 This work is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by- sa/3.0/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Fran- cisco, California, 94105, USA. 3 Introduction A holiday on the Bawley Coast is still just like it was for our grandparents in their youth. Simple, sunny, lazy, memorable. Regenerating. This publication gives an over- view of the Bawley Coast area, its history and its present. There is also information about our coastal retreat, Bawley Bush Cottages. We hope this guide improves your ex- perience of this beautiful piece of Australian coast and hinterland. Here on the Bawley Coast geological history, evidence of aboriginal occupation and the more recent settler past are as easily visible as our beaches, lakes and cafes. Our coast remains relatively unspoiled by many of the trappings of town life. Recreation, sustain- ability and environmental protection are our high priorities. Landcare, dunecare and the protection of endangered species are examples of environ- mental activities important to our community. -

Government Gazette of the STATE of NEW SOUTH WALES Number 26 Friday, 29 February 2008 Published Under Authority by Government Advertising
1253 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 26 Friday, 29 February 2008 Published under authority by Government Advertising LEGISLATION Proclamations New South Wales Commencement Proclamation under the Classification (Publications, Films and Computer Games) Enforcement Amendment Act 2007 No 60 MARIE BASHIR,, GovernorGovernor I, Professor Marie Bashir AC, CVO, Governor of the State of New South Wales, with the advice of the Executive Council, and in pursuance of section 2 (1) of the Classification (Publications, Films and Computer Games) Enforcement Amendment Act 2007, do, by this my Proclamation, appoint 16 March 2008 as the day on which that Act, except Schedule 1 [6], commences. Signed and sealed at Sydney, thisthis 20th day of February day of 2008. 2008. By Her Excellency’s Command, JOHN HATZISTERGOS, M.L.C., L.S. AttorneyAttorney GeneralGeneral GOD SAVE THE QUEEN! Explanatory note The object of this Proclamation is to commence the majority of the provisions of the Classification (Publications, Films and Computer Games) Enforcement Amendment Act 2007, including provisions consequent on the enactment of amendments to the Classification (Publications, Films and Computer Games) Amendment Act 2007 of the Commonwealth (the corresponding Commonwealth Act), and provisions relating to the giving of exemptions under the Classification (Publications, Films and Computer Games) Enforcement Act 1995. The uncommenced provision (Schedule 1 [6]) commences when relevant amendments to the corresponding Commonwealth Act commence. s2008-011-30.d05 -

Historical Riparian Vegetation Changes in Eastern NSW
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2016 Historical Riparian Vegetation Changes in Eastern NSW Angus Skorulis Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Skorulis, Angus, Historical Riparian Vegetation Changes in Eastern NSW, BSci Hons, School of Earth & Environmental Science, University of Wollongong, 2016. https://ro.uow.edu.au/thsci/120 Research Online is the open access institutional repository for the University of Wollongong. -

Murramarang South Coast Walk (NPWS Estate)
Murramarang South Coast Walk (NPWS Estate) Consultation Draft Review of Environmental Factors November 2019 File: 16 July 2020 To whom it may concern Subject: Murramarang South Coast Walk Draft Review of Environmental Factors (REF) This draft REF describes the potential impacts of the proposed Murramarang South Coast Walk on the environment and details safeguard and mitigation measures to be implemented. This draft REF was prepared before the Curowan Fire which burnt much of the Murramarang National Park over the 2019/20 summer. The landscape is adapted to fire and will regenerate, however, we now have an opportunity to undertake additional targeted environmental surveys and identify habitat constraints that may have changed. We have also engaged a consultant to work with the Aboriginal community to survey the proposed development area and prepare an Aboriginal Heritage Impact Permit (AHIP). As this work was also done before the Curowan Fire, more cultural heritage surveys and assessments will need to be undertaken. This draft REF will be updated to incorporate the outcomes of the post-fire environmental and cultural heritage assessments and the public consultation before being submitted to the Department of Planning, Industry, and Environment (DPIE) to decide whether the Murramarang South Coast Walk should go ahead. DPIE will also assist in the development of appropriate mitigation measures should approval be given. The REF will be available for public comment until Sunday 9 August 2020. Your comments will help identify the actions required -

Sydneyœsouth Coast Region Irrigation Profile
SydneyœSouth Coast Region Irrigation Profile compiled by Meredith Hope and John O‘Connor, for the W ater Use Efficiency Advisory Unit, Dubbo The Water Use Efficiency Advisory Unit is a NSW Government joint initiative between NSW Agriculture and the Department of Sustainable Natural Resources. © The State of New South Wales NSW Agriculture (2001) This Irrigation Profile is one of a series for New South Wales catchments and regions. It was written and compiled by Meredith Hope, NSW Agriculture, for the Water Use Efficiency Advisory Unit, 37 Carrington Street, Dubbo, NSW, 2830, with assistance from John O'Connor (Resource Management Officer, Sydney-South Coast, NSW Agriculture). ISBN 0 7347 1335 5 (individual) ISBN 0 7347 1372 X (series) (This reprint issued May 2003. First issued on the Internet in October 2001. Issued a second time on cd and on the Internet in November 2003) Disclaimer: This document has been prepared by the author for NSW Agriculture, for and on behalf of the State of New South Wales, in good faith on the basis of available information. While the information contained in the document has been formulated with all due care, the users of the document must obtain their own advice and conduct their own investigations and assessments of any proposals they are considering, in the light of their own individual circumstances. The document is made available on the understanding that the State of New South Wales, the author and the publisher, their respective servants and agents accept no responsibility for any person, acting on, or relying on, or upon any opinion, advice, representation, statement of information whether expressed or implied in the document, and disclaim all liability for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information contained in the document or by reason of any error, omission, defect or mis-statement (whether such error, omission or mis-statement is caused by or arises from negligence, lack of care or otherwise). -

NPWS Annual Report 2000-2001 (PDF
Annual report 2000-2001 NPWS mission NSW national Parks & Wildlife service 2 Contents Director-General’s foreword 6 3 Conservation management 43 Working with Aboriginal communities 44 Overview 8 Joint management of national parks 44 Mission statement 8 Performance and future directions 45 Role and functions 8 Outside the reserve system 46 Partners and stakeholders 8 Voluntary conservation agreements 46 Legal basis 8 Biodiversity conservation programs 46 Organisational structure 8 Wildlife management 47 Lands managed for conservation 8 Performance and future directions 48 Organisational chart 10 Ecologically sustainable management Key result areas 12 of NPWS operations 48 Threatened species conservation 48 1 Conservation assessment 13 Southern Regional Forest Agreement 49 NSW Biodiversity Strategy 14 Caring for the environment 49 Regional assessments 14 Waste management 49 Wilderness assessment 16 Performance and future directions 50 Assessment of vacant Crown land in north-east New South Wales 19 Managing our built assets 51 Vegetation surveys and mapping 19 Buildings 51 Wetland and river system survey and research 21 Roads and other access 51 Native fauna surveys and research 22 Other park infrastructure 52 Threat management research 26 Thredbo Coronial Inquiry 53 Cultural heritage research 28 Performance and future directions 54 Conservation research and assessment tools 29 Managing site use in protected areas 54 Performance and future directions 30 Performance and future directions 54 Contributing to communities 55 2 Conservation planning -

Review of State Conservation Areas
Review of State Conservation Areas Report of the first five-year review of State Conservation Areas under the National Parks and Wildlife Act 1974 November 2008 Cover photos (clockwise from left): Trial Bay Goal, Arakoon SCA (DECC); Glenrock SCA (B. Peters, DECC); Banksia, Bent Basin SCA (M. Lauder, DECC); Glenrock SCA (B. Peters, DECC). © Copyright State of NSW and Department of Environment and Climate Change NSW. The Department of Environment and Climate Change NSW and State of NSW are pleased to allow this material to be reproduced for educational or non-commercial purposes in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. Specific permission is required for the reproduction of photographs. Published by: Department of Environment and Climate Change 59–61 Goulburn Street PO Box A290 Sydney South 1232 Ph: (02) 9995 5000 (switchboard) Ph: 131 555 (environment information and publications requests) Ph: 1300 361 967 (national parks information and publications requests) Fax: (02) 9995 5999 TTY: (02) 9211 4723 Email: [email protected] Website: www.environment.nsw.gov.au ISBN 978-1-74122-981-3 DECC 2008/516 November 2008 Printed on recycled paper Contents Minister’s Foreword iii Part 1 – State Conservations Areas 1 State Conservation Areas 4 Exploration and mining in NSW 6 History and current trends 6 Titles 7 Assessments 7 Compliance and rehabilitation 8 Renewals 8 Exploration and mining in State Conservation Areas 9 The five-year review 10 Purpose of the review 10 -

Nexusmagazine.Com
N E X U S NEW TIMES MAGAZINE Volume 14, Number 3 APRIL – MAY 2007 UK/Europe edition Website: http://www.nexusmagazine.com LETTERS TO THE EDITOR.............................................4 THE CRIMINAL HISTORY OF THE PAPACY – Pt 3.....49 Comments from readers on NEXUS-related topics. By Tony Bushby. Modern Roman Catholic Church GLOBAL NEWS.............................................................6 historians hide the bellicosity, depravity and greed We report on declining media freedom in the USA, of so many of the popes and instead present images a study linking GM potato consumption to cancer, of pious and humble patriarchs of the people. and the soaring death rate from prescription drugs. C L O S E E N C O U N T E R S W I T H " L I T T L E F O O T " . 5 7 E M W E A P O N S A N D H U M A N R I G H T S . 11 By Tony Healy and Paul Cropper. Little hairy ape- By Peter Phillips, Lew Brown and Bridget Thornton. men or "junjudees" have been seen and reported by The US military-industrial-intelligence complex is Aborigines and new Australians up to the present armed with an array of electromagnetic weapons, day, and they may have Flores "hobbit" roots. ready to be deployed against inconvenient THE TWILIGHT ZONE................................................63 dissenters and in contravention of civil rights. Our "out there" news this edition features the second PO M E G R A N AT E : F R U I T O F T H E T R E E O F L I F E . -

Part B Chapter 10.2 WATER REFORM NEW SOUTH WALES NCP
NCP second tranche Assessment Water: New South Wales B10.2 WATER REFORM, NEW SOUTH WALES ASSESSMENT, June 1999 Page Table of Contents 278 Table of abbreviations 280 B10.2.1 EXECUTIVE SUMMARY 283 B10.2.2 REFORM COMMITMENT: COST REFORM AND PRICING 287 10.2.2.1 Cost Recovery 287 10.2.2.2 Consumption Based Pricing 294 10.2.2.3 Cross Subsidies 298 10.2.2.4 CSOs 301 10.2.2.5 Rates of Return 301 10.2.2.6 Rural Cost Recovery 302 10.2.2.7 New Rural Schemes 303 10.2.2.8 Devolution of Irrigation Management 304 B10.2.3 REFORM COMMITMENT: INSTITUTIONAL REFORM 305 10.2.3.1 Separation of Functions 305 10.2.3.2 Commercial Focus 310 10.2.3.3 Performance Monitoring and Best Practice 311 B10.2.4 REFORM COMMITMENT: ALLOCATION AND TRADING 313 10.2.4.1 Water Entitlements 313 10.2.4.2 Environmental Allocations 319 10.2.4.3 Water Trading 331 B10.2.5 REFORM COMMITMENT: ENVIRONMENT AND WATER QUALITY 336 10.2.5.1 Integrated Catchment Management 336 10.2.5.2 National Water Quality Management Strategy 340 278 NCP second tranche Assessment Water: New South Wales B10.2.6 REFORM COMMITMENT: PUBLIC CONSULTATION, EDUCATION 343 ATTACHMENTS 345 Attachment 1: Table of cost recovery for NMUs with more than 10 000 connections Attachment 2: Tariff structures for NMUs with more than 10 000 connections Attachment 3: New South Wales allocation and trading implementation program Attachment 4: Unregulated catchments Attachment 5: Groundwater 279 NCP second tranche Assessment Water: New South Wales T a b l e o f Ab b r e v i a t i o n s ARMCANZ Agriculture and Resource Management -
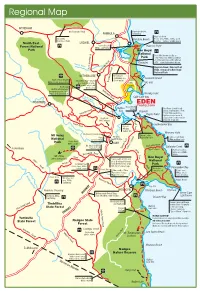
Regional Map
Regional Map WYNDHAM Mt Darragh Road Barmouth Beach PAMBULA Swimming Haycock Point Goodenia Pambula Beach Picnic area, BBQs, toilets, good Rainforest Walk beaches – fishing, SCUBA diving South East LOCHIEL Severs Beach Haycock Point Forest National BBQs, swimming Park Ben Boyd National Red cliffs (known locally as Park “The Pinnacles) Striking contrast of white sandstone cliffs with red cliffs – a photoghraphers dream Yowaka River Pinnacles Haycock Road, 8km north of Back Creek Road Eden, entrance to Ben Boyd Nethercote Road National Park NETHERCOTE Broadwater swimming area Leonards Island Nullica River Mouth Quarantine Bay – 4 lane (fresh water) Broadwater Road BBQs, picnic area, toilets boat launching ramp, Lake 4WD ONLY ONLY public wharf and Curalo Boydtown picnic area Built by whaling king Nethercote Road Benjamin Boyd in 1843 Ruins of Boyds Church Worang Point Nullica River Calle Calle Bay TOWAMBA EDEN TWOFOLD BAY Nullica Navy Wharf fishingTWOFOLD BAY Red Point (South Head) Bay Chipmill Boyds Tower built in 1846 Towamba Road 19.5m high sandstone Boyds Tower lighthouse was never lit, Boydtown steps down to observation platform down cliff Hill Cottages Boyd Snake Track Road Leatherjacket Bay Towamba River Historic Whaling station site Mowarry Point Kiah Country Gardens Mt Imlay Edrom Lodge Kiah Whelans Built 1910-1913 Light to Light Walk National General Store Swamp Bridge 30km walking track Park Picnic Area BBQs, water KIAH Mt. Imlay walking Duck Hole Road Saltwater Creek Edrom Road To Bombala track turn off 15km to Chipmiill Green GRAVEL ROAD Fishing, beaches, Road picnic area, BBQs, Burrawang Cape toilets Mt. Imlay Road Ben Boyd Imlay Road 886m walking track 19km south of Eden turn off to Ben Boyd National National Anteater Park, Boyds Tower, Chip Scrubby Creek Road Park Picnic Area, BBQs, Mill, Edrom, Greencape Wonboyn Bittangabee Bay toilets, water Picnic Area 23km south of Eden Lake Resort Picnic area, Turn off to Nadgee BBQs, toilets, Wallagaruagh River Nature Reserve ruins, beaches Picnic Area Mt.