Mitochondrial Dynamics in Hematopoietic Stem Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Characterization of Dysregulated Lncrna-Mrna Network Based on Cerna Hypothesis to Reveal the Occurrence and Recurrence of Myocar
Zhang et al. Cell Death Discovery (2018) 4:35 DOI 10.1038/s41420-018-0036-7 Cell Death Discovery ARTICLE Open Access Characterization of dysregulated lncRNA- mRNA network based on ceRNA hypothesis to reveal the occurrence and recurrence of myocardial infarction Guangde Zhang1,HaoranSun2, Yawei Zhang2, Hengqiang Zhao2, Wenjing Fan1,JianfeiLi3,YingliLv2, Qiong Song2, Jiayao Li2,MingyuZhang1 and Hongbo Shi2 Abstract Accumulating evidence has demonstrated that long non-coding RNAs (lncRNAs) acting as competing endogenous RNAs (ceRNAs) play important roles in initiation and development of human diseases. However, the mechanism of ceRNA regulated by lncRNA in myocardial infarction (MI) remained unclear. In this study, we performed a multi-step computational method to construct dysregulated lncRNA-mRNA networks for MI occurrence (DLMN_MI_OC) and recurrence (DLMN_MI_Re) based on “ceRNA hypothesis”. We systematically integrated lncRNA and mRNA expression profiles and miRNA-target regulatory interactions. The constructed DLMN_MI_OC and DLMN_MI_Re both exhibited biological network characteristics, and functional analysis demonstrated that the networks were specific for MI. Additionally, we identified some lncRNA-mRNA ceRNA modules involved in MI occurrence and recurrence. Finally, two new panel biomarkers defined by four lncRNAs (RP1-239B22.5, AC135048.13, RP11-4O1.2, RP11-285F7.2) from 1234567890():,; 1234567890():,; DLMN_MI_OC and three lncRNAs (RP11-363E7.4, CTA-29F11.1, RP5-894A10.6) from DLMN_MI_Re with high classification performance were, respectively, identified in distinguishing controls from patients, and patients with recurrent events from those without recurrent events. This study will provide us new insight into ceRNA-mediated regulatory mechanisms involved in MI occurrence and recurrence, and facilitate the discovery of candidate diagnostic and prognosis biomarkers for MI. -

Germline Mutations Causing Familial Lung Cancer
Journal of Human Genetics (2015) 60, 597–603 & 2015 The Japan Society of Human Genetics All rights reserved 1434-5161/15 www.nature.com/jhg ORIGINAL ARTICLE Germline mutations causing familial lung cancer Koichi Tomoshige1,2, Keitaro Matsumoto1, Tomoshi Tsuchiya1, Masahiro Oikawa1, Takuro Miyazaki1, Naoya Yamasaki1, Hiroyuki Mishima2, Akira Kinoshita2, Toru Kubo3, Kiyoyasu Fukushima3, Koh-ichiro Yoshiura2 and Takeshi Nagayasu1 Genetic factors are important in lung cancer, but as most lung cancers are sporadic, little is known about inherited genetic factors. We identified a three-generation family with suspected autosomal dominant inherited lung cancer susceptibility. Sixteen individuals in the family had lung cancer. To identify the gene(s) that cause lung cancer in this pedigree, we extracted DNA from the peripheral blood of three individuals and from the blood of one cancer-free control family member and performed whole-exome sequencing. We identified 41 alterations in 40 genes in all affected family members but not in the unaffected member. These were considered candidate mutations for familial lung cancer. Next, to identify somatic mutations and/or inherited alterations in these 40 genes among sporadic lung cancers, we performed exon target enrichment sequencing using 192 samples from sporadic lung cancer patients. We detected somatic ‘candidate’ mutations in multiple sporadic lung cancer samples; MAST1, CENPE, CACNB2 and LCT were the most promising candidate genes. In addition, the MAST1 gene was located in a putative cancer-linked locus in the pedigree. Our data suggest that several genes act as oncogenic drivers in this family, and that MAST1 is most likely to cause lung cancer. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Rabbit Anti-FCRL1 Antibody-SL16064R
SunLong Biotech Co.,LTD Tel: 0086-571- 56623320 Fax:0086-571- 56623318 E-mail:[email protected] www.sunlongbiotech.com Rabbit Anti-FCRL1 antibody SL16064R Product Name: FCRL1 Chinese Name: CD307a抗体 CD307a; Fc receptor homolog 1; Fc receptor like protein 1; Fc receptor-like protein 1; FcR like protein 1; FcR-like protein 1; FCRH 1; FcRH1; FCRL 1; FcRL1; FCRL1_HUMAN; hIFGP 1; hIFGP1; IFGP 1; IFGP family protein 1; IFGP1; Immune Alias: receptor translocation associated protein 5; Immune receptor translocation-associated protein 5; immunoglobulin superfamily Fc receptor, gp42; IRTA 5; IRTA5; RP11 367J7.7. Organism Species: Rabbit Clonality: Polyclonal React Species: Human, WB=1:500-2000ELISA=1:500-1000IHC-P=1:400-800IHC-F=1:400-800ICC=1:100- 500IF=1:100-500(Paraffin sections need antigen repair) Applications: not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. Molecular weight: 45kDa Cellular localization: The cell membrane Form: Lyophilizedwww.sunlongbiotech.com or Liquid Concentration: 1mg/ml KLH conjugated synthetic peptide derived from human FCRL1:251- immunogen: 350/429<Extracellular> Lsotype: IgG Purification: affinity purified by Protein A Storage Buffer: 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. The lyophilized antibody is stable at room temperature for at least one month and for greater than a year Storage: when kept at -20°C. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. -

Single-Cell Transcriptome Profiling of the Kidney Glomerulus Identifies Key Cell Types and Reactions to Injury
BASIC RESEARCH www.jasn.org Single-Cell Transcriptome Profiling of the Kidney Glomerulus Identifies Key Cell Types and Reactions to Injury Jun-Jae Chung ,1 Leonard Goldstein ,2 Ying-Jiun J. Chen,2 Jiyeon Lee ,1 Joshua D. Webster,3 Merone Roose-Girma,2 Sharad C. Paudyal,4 Zora Modrusan,2 Anwesha Dey,5 and Andrey S. Shaw1 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background The glomerulus is a specialized capillary bed that is involved in urine production and BP control. Glomerular injury is a major cause of CKD, which is epidemic and without therapeutic options. Single-cell transcriptomics has radically improved our ability to characterize complex organs, such as the kidney. Cells of the glomerulus, however, have been largely underrepresented in previous single-cell kidney studies due to their paucity and intractability. Methods Single-cell RNA sequencing comprehensively characterized the types of cells in the glomerulus from healthy mice and from four different disease models (nephrotoxic serum nephritis, diabetes, doxo- rubicin toxicity, and CD2AP deficiency). Results Allcelltypesintheglomeruluswereidentified using unsupervised clustering analysis. Novel marker genes and gene signatures of mesangial cells, vascular smooth muscle cells of the afferent and efferent arteri- oles, parietal epithelial cells, and three types of endothelial cells were identified. Analysis of the disease models revealed cell type–specific and injury type–specific responses in the glomerulus, including acute activation of the Hippo pathway in podocytes after nephrotoxic immune injury. Conditional deletion of YAP or TAZ resulted in more severe and prolonged proteinuria in response to injury, as well as worse glomerulosclerosis. -

Genome-Wide DNA Methylation Dynamics During Epigenetic
Gómez‑Redondo et al. Clin Epigenet (2021) 13:27 https://doi.org/10.1186/s13148‑021‑01003‑x RESEARCH Open Access Genome‑wide DNA methylation dynamics during epigenetic reprogramming in the porcine germline Isabel Gómez‑Redondo1*† , Benjamín Planells1†, Sebastián Cánovas2,3, Elena Ivanova4, Gavin Kelsey4,5 and Alfonso Gutiérrez‑Adán1 Abstract Background: Prior work in mice has shown that some retrotransposed elements remain substantially methylated during DNA methylation reprogramming of germ cells. In the pig, however, information about this process is scarce. The present study was designed to examine the methylation profles of porcine germ cells during the time course of epigenetic reprogramming. Results: Sows were artifcially inseminated, and their fetuses were collected 28, 32, 36, 39, and 42 days later. At each time point, genital ridges were dissected from the mesonephros and germ cells were isolated through magnetic‑ activated cell sorting using an anti‑SSEA‑1 antibody, and recovered germ cells were subjected to whole‑genome bisulphite sequencing. Methylation levels were quantifed using SeqMonk software by performing an unbiased analysis, and persistently methylated regions (PMRs) in each sex were determined to extract those regions showing 50% or more methylation. Most genomic elements underwent a dramatic loss of methylation from day 28 to day 36, when the lowest levels were shown. By day 42, there was evidence for the initiation of genomic re‑methylation. We identifed a total of 1456 and 1122 PMRs in male and female germ cells, respectively, and large numbers of transpos‑ able elements (SINEs, LINEs, and LTRs) were found to be located within these PMRs. Twenty‑one percent of the introns located in these PMRs were found to be the frst introns of a gene, suggesting their regulatory role in the expression of these genes. -

Supplemental Data
SUPPLEMENTAL DATA A transcriptomic continuum of differentiation arrest identifies myeloid interface acute leukemias with poor prognosis Bond et al Contents: • Supplementary Table Legends • Supplementary Tables S6, S8 - S11 • Supplementary Figures S1 - S4 • Supplementary Methods • Supplementary References Supplementary Table Legends: Supplementary Table S1: Gene-sets used for GSEA analysis in this study (see Excel file). Supplementary Table S2: Details of patient cohort (see Excel file). Supplementary Table S3: Differential gene expression analysis comparing AML-like T- ALLs with other T-ALLs (see Excel file). Positive values denote genes with higher expression in AML-like T-ALLs. Supplementary Table S4: Differential gene expression analysis for the comparisons of thymic subset populations indicated in each tab (see Excel files). Supplementary Table S5: ICGS output (see Excel file). First row contains sample names, second row the ICGS clusters, and the following rows guide genes and their normalized expression. The second column indicates the guide genes groups as indicated by the black and white bars in Figure 2A (first tab), Supplementary Figure S3A (second tab (1)) and S3C (third tab (2)). Supplementary Table S6: Genes included in the targeted NGS panel. Supplementary Table S7: Mutational status by NGS (see Excel file). 0 = no mutation, 1 = mutation of known significance, 2 = mutation of unknown significance. Supplementary Table S8: List of genes used for IAL score Supplementary Table S9: Impact of IAL score on outcome according to ELN subgroup. Supplementary Table S10: Comparison of clinicobiological characteristics and mutational profiles of cases with high and low IAL scores in the ALFA-0701 cohort (3). Supplemental Table S11: Univariate analyses of Overall Survival in the ALFA-0701 cohort (3). -
Primepcr™Assay Validation Report
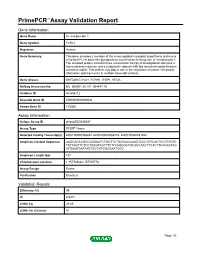
PrimePCR™Assay Validation Report Gene Information Gene Name Fc receptor-like 1 Gene Symbol FCRL1 Organism Human Gene Summary This gene encodes a member of the immunoglobulin receptor superfamily and is one of several Fc receptor-like glycoproteins clustered on the long arm of chromosome 1. The encoded protein contains three extracellular C2-like immunoglobulin domains a transmembrane domain and a cytoplasmic domain with two immunoreceptor-tyrosine activation motifs. This protein may play a role in the regulation of cancer cell growth. Alternative splicing results in multiple transcript variants. Gene Aliases DKFZp667O1421, FCRH1, IFGP1, IRTA5 RefSeq Accession No. NC_000001.10, NT_004487.19 UniGene ID Hs.656112 Ensembl Gene ID ENSG00000163534 Entrez Gene ID 115350 Assay Information Unique Assay ID qHsaCED0046097 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000358292, ENST00000368176, ENST00000491942 Amplicon Context Sequence AAGGAGCCGGCAGGAATCTGGTTCTGATAACAAAGTCCCTGTCACTCCTGTGTC TATTAGTTCTCCTAGGTAGTTTCTTCAGGGCTGCGCCAACTTCACTTCACAGTAG ATGAAGGAATAGTGCCATGGAGAATGGC Amplicon Length (bp) 107 Chromosome Location 1:157765642-157765778 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 99 R2 0.9971 cDNA Cq 29.05 cDNA Tm (Celsius) 81 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq 25.04 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report FCRL1, Human Amplification Plot Amplification of cDNA generated from 25 ng -

A Single Genetic Locus Associated with Pediatric Fractures: a Genome-Wide Association Study on 3,230 Patients
1716 EXPERIMENTAL AND THERAPEUTIC MEDICINE 20: 1716-1724, 2020 A single genetic locus associated with pediatric fractures: A genome-wide association study on 3,230 patients ROOPE PARVIAINEN1, SINI SKARP2,3, LINDA KORHONEN1, WILLY SERLO1, MINNA MÄNNIKKÖ2 ��� JUHA-JAAKKO SINIKUMPU1 1D�p���m��� �� C������� ��� A����������, O��� C�������� F������� ��� Sp���� I�j��y S���y, R������� U��� ��� P���������, P�������� N�������y, P�������� Surgery, C���� P�y������y, D��m������y, C������� G�������, O��������� ��� Gy�������y, O����������y������y ��� Op�����m����y (PEDEGO), O��� M������ R������� C����� (MRC), U��������y �� O��� ��� O��� U��������y H��p����, FI-90029 O���; 2N������� F������ B���� C�����, F�����y �� M�������, U��������y �� O���; 3C����� ��� L��� C����� H����� R�������, F�����y �� M�������, U��������y �� O���, FI-90014 O���, F������ Received July 13, 2019; Accepted April 29, 2020 DOI: 10.3892/etm.2020.8885 Abstract. The�� understanding������������� ��of the��� biological���������� and��� environ�������- RNA 1 (PROSER2-AS1) and PROSER2, thus suggesting that mental risk factors of fractures in pediatrics is limited. Previous these m�y be novel candidate genes associated with the risk of studies have reported that fractures involve heritable traits, but pediatric fractures. the genetic factors contributing to the risk of fractures remain elusive. Furthermore, genetic influences specific to immature Introduction bone have not been thoroughly studied. Therefore, the aim of the present study was to identify genetic variations that are The incidence of fractures is highest �mong the young and associated with fractures in early childhood. The present study older p�pulations (1). O� note, fractures in pediatric patients used � prospective Northern Finland Birth Cohort (year 1986; are common injuries that result in pain, as well as short‑ and �=9,432). -

Cis-Regulatory Differences Explaining Evolved Levels of Endometrial Invasibility in Eutherian Mammals
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.04.283366; this version posted September 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Cis-Regulatory Differences Explaining Evolved Levels of Endometrial Invasibility in Eutherian Mammals Yasir Suhail1,3, Jamie D. Maziarz2,3, Anasuya Dighe 2,3 , Gunter Wagner2,3,5,6,*, Kshitiz1,3,4,* 1 Department of Biomedical Engineering, University of Connecticut Health, Farmington, CT 2. Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 3. Cancer Systems Biology Center (CaSB@Yale), Yale University, West Haven, CT 4. Center for Cell Analysis and Modeling, University of Connecticut Health, Farmington, CT 4. Department of Obstetrics, Gynecology and Reproductive Sciences, Yale Medical School, New Haven, CT 5. Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI * Corresponding Authors: [email protected], [email protected] Abstract Eutherian (placental) mammals exhibit great differences in the degree of placental invasion into the maternal endometrium, with humans being on the most invasive end. Previously, we have shown that these differences in invasiveness is largely controlled by the stromal fibroblasts of the maternal endometrium, with secondary effect on stroma of other tissues resulting in correlated differences in cancer malignancy. Here, we present a statistical investigation of the second dogma linking the phenotypic and transcriptional differences to the genomic changes across species, revealing the regulatory genomic sequence differences underlying these inter-species differences.