Kidney – Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Renal Aquaporins
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 49 (1996), pp.1712—1717 Renal aquaporins MARK A. KNEPPER, JAMES B. WADE, JAMES TERRIS, CAROLYN A. ECELBARGER, DAVID MARPLES, BEATRICE MANDON, CHUNG-LIN CHOU, B.K. KISHORE, and SØREN NIELSEN Laborato,y of Kidney and Electrolyte Metabolism, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Matyland, USA; Department of Cell Biology, Institute of Anatomy, University of Aarhus, Aarhus, Denmark; and Department of Physiology, University of Maiyland College of Medicine, Baltimore, and Department of Physiology, Unifornied Services University of the Health Sciences, Bethesda, Maiyland, USA Renal aquaporins. Aquaporins (AQPs) are a newly recognized family of gate the localization and regulation of the four renal aquaporins transmembrane proteins that function as molecular water channels. At (AQP1, AQP2, AQP3 and AQP4). least four aquaporins are expressed in the kidney where they mediate Urine is concentrated as a result of the combined function of rapid water transport across water-permeable epithelia and play critical roles in urinary concentrating and diluting processes. AQP1 is constitu- the loop of Henle, which generates a high osmolality in the renal tively expressed at extremely high levels in the proximal tubule and medulla by countercurrent multiplication, and the collecting duct, descending limb of Henle's loop. AQP2, -3 and -4 are expressed predom- which, in the presence of the antidiuretic hormone vasopressin, inantly in the collecting duct system. AQP2 is the predominant water permits osmotic equilibration between the urine and the hyper- channel in the apical plasma membrane and AQP3 and -4arefound in the basolateral plasma membrane. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -

Kaplan USMLE Step 1 Prep: Distribution Ion Channel Protein in Kidney
Kaplan USMLE Step 1 prep: Distribution ion channel protein in kidney FEB 3, 2020 Staff News Writer If you’re preparing for the United States Medical Licensing Examination® (USMLE®) Step 1 exam, you might want to know which questions are most often missed by test-prep takers. Check out this example from Kaplan Medical, and read an expert explanation of the answer. Also check out all posts in this series. This month’s stumper An investigator is examining the distribution of an ion channel protein in the kidney. Slices of kidney tissue are incubated in a dilute solution of a specific antibody directed against the protein. The immunoperoxidase method is then used to localize the ion channel proteins. In one area, the investigator notes epithelial cells with a brush border that are positive for the ion channel protein. Which of the following areas is most likely to show these microscopic characteristics? A. Collecting duct. B. Descending thin limb of the loop of Henle. C. Distal convoluted tubule. D. Glomerulus. E. Proximal convoluted tubule. URL: https://www.ama-assn.org/residents-students/usmle/kaplan-usmle-step-1-prep-distribution-ion-channel-protein- kidney Copyright 1995 - 2021 American Medical Association. All rights reserved. The correct answer is E. Kaplan Medical explains why The proximal convoluted tubule (PCT) is the only portion of the renal tubule in which the epithelial cells have a "brush border." The brush border is composed of microvilli, which greatly increases apical membrane surface area and thereby enhances epithelial reabsorptive capacity. The PCT recovers almost 100% of filtered organic solutes (e.g., glucose, amino acids, proteins) and about 67% of electrolytes and water, amounting to about 120 L of the daily filtered load. -
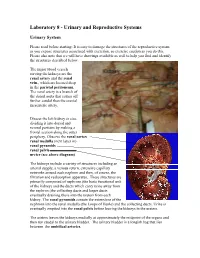
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Renal Physiology and Pathophysiology of the Kidney
Renal Physiology and pathophysiology of the kidney Alain Prigent Université Paris-Sud 11 IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 The glomerular filtration rate (GFR) may change with - The adult age ? - The renal plasma (blood) flow ? + - The Na /water reabsorption in the nephron ? - The diet variations ? - The delay after a kidney donation ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 GFR can measure with the following methods - The Cockcroft-Gault formula ? - The urinary creatinine clearance ? - The Counahan-Baratt method in children? - The Modification on Diet in Renal Disease (MDRD) formula in adults ? - The MAG 3 plasma sample clearance ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 About the determinants of the renogram curve (supposed to be perfectly « BKG » corrected) 99m -The uptake (initial ascendant segment) of Tc DTPA depends on GFR 99m -The uptake (initial ascendant segment) of Tc MAG 3 depends almost only on renal plasma flow 123 -The uptake (initial ascendant segment) of I hippuran depends both on renal plasma flow and GFR -The height of renogram maximum (normalized to the injected activity) reflects on the total nephron number -The « plateau » pattern of the late segment of the renogram does mean obstruction ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 Overview of the kidney functions Regulation of the volume and composition of the body fluids -

Urine-Derived Epithelial Cells As Models for Genetic Kidney Diseases
cells Review Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases Tjessa Bondue 1 , Fanny O. Arcolino 1 , Koenraad R. P. Veys 1,2, Oyindamola C. Adebayo 1,3, Elena Levtchenko 1,2, Lambertus P. van den Heuvel 1,4 and Mohamed A. Elmonem 5,* 1 Department of Development and Regeneration, KU Leuven, 3000 Leuven, Belgium; [email protected] (T.B.); [email protected] (F.O.A.); [email protected] (K.R.P.V.); [email protected] (O.C.A.); [email protected] (E.L.); [email protected] (L.P.v.d.H.) 2 Department of Pediatrics, Division of Pediatric Nephrology, University Hospitals Leuven, 3000 Leuven, Belgium 3 Centre for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, 3000 Leuven, Belgium 4 Department of Pediatric Nephrology, Radboud University Medical Center, 6500 Nijmegen, The Netherlands 5 Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Cairo 11628, Egypt * Correspondence: [email protected] Abstract: Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much Citation: Bondue, T.; Arcolino, F.O.; interest. In this review, we summarize the methodology for establishing human podocyte and PTEC Veys, K.R.P.; Adebayo, O.C.; cell lines from urine and highlight their importance as kidney disease cell models. -

URINARY SYSTEM Components
Human Anatomy Unit 3 URINARY SYSTEM Components • Kidneys • Ureters • Urinary bladder • Urethra Funcons • Storage of urine – Bladder stores up to 1 L of urine • Excreon of urine – Transport of urine out of body • Regulaon: – Plasma pH – Blood volume/pressure – Plasma ion concentraons (Ca2+, Na+, K+, CL-) – Assist liver in detoxificaon, amino acid metabolism Kidney Gross Anatomy • Retroperitoneal – Anterior surface covered with peritoneum – Posterior surface directly against posterior abdominal wall • Superior surface at about T12 • Inferior surface at about L3 • Ureters enter urinary bladder posteriorly • LeT kidney 2cm superior to right – Size of liver Structure of the Kidney • Hilum = the depression along the medial border through which several structures pass – renal artery – renal vein – ureter – renal nerves Surrounding Tissue • Fibrous capsule – Innermost layer of dense irregular CT – Maintains shape, protec:on • Adipose capsule – Adipose ct of varying thickness – Cushioning and insulaon • Renal fascia – Dense irregular CT – Anchors kidney to peritoneum & abdominal wall • Paranephric fat – Outermost, adipose CT between renal fascia and peritoneum Frontal Sec:on of the Kidney • Cortex – Layer of renal :ssue in contact with capsule – Renal columns – parts of cortex that extend into the medulla between pyramids • Medulla – Striped due to renal tubules • Renal pyramids – 8-15 present in medulla of adult – Conical shape – Wide base at cor:comedullary juncon Flow of Filtrate/Urine • Collec:ng ducts – Collect from mul:ple nephrons • Minor calyx – Collect from each pyramid • Major calyx – Collect from minor calyx • Renal pelvis – Collects from calyces, passes onto • Ureter – Collects from pelvis • Urinary Bladder – Collects from ureters Histology Renal Cortex Renal Medulla Renal Tubules • Nephron – func:onal unit of the kidney. -

Different Localization and Regulation of Two Types of Vasopressin Receptor
Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. Y Terada, … , T Yang, F Marumo J Clin Invest. 1993;92(5):2339-2345. https://doi.org/10.1172/JCI116838. Research Article Recent studies have revealed that arginine vasopressin (AVP) has at least two types of receptors in the kidney: V1a receptor and V2 receptor. In this study, microlocalization of mRNA coding for V1a and V2 receptors was carried out in the rat kidney using a reverse transcription and polymerase chain reaction. Large signals for V1a receptor PCR product were detected in the glomerulus, initial cortical collecting duct, cortical collecting duct, outer medullary collecting duct, inner medullary collecting duct, and arcuate artery. Small but detectable signals were found in proximal convoluted and straight tubules, inner medullary thin limbs, and medullary thick ascending limbs. Large signals for V2 receptor mRNA were detected in the cortical collecting duct, outer medullary collecting duct, and inner medullary collecting duct. Small signals for V2 receptor were found in the inner medullary thick limbs, medullary thick ascending limbs, and initial cortical collecting duct. Next, we investigated V1a and V2 receptor mRNA regulation in the dehydrated state. During a 72-h water restriction state, the plasma AVP level increased and V2 receptor mRNA decreased in collecting ducts. In contrast, V1a receptor mRNA did not change significantly. Thus, the