The Stubenberg Meteorite - an LL6 Chondrite Fragmental Breccia Recovered Soon After Precise Prediction of the Strewn Field
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Handbook of Iron Meteorites, Volume 3
Sierra Blanca - Sierra Gorda 1119 ing that created an incipient recrystallization and a few COLLECTIONS other anomalous features in Sierra Blanca. Washington (17 .3 kg), Ferry Building, San Francisco (about 7 kg), Chicago (550 g), New York (315 g), Ann Arbor (165 g). The original mass evidently weighed at least Sierra Gorda, Antofagasta, Chile 26 kg. 22°54's, 69°21 'w Hexahedrite, H. Single crystal larger than 14 em. Decorated Neu DESCRIPTION mann bands. HV 205± 15. According to Roy S. Clarke (personal communication) Group IIA . 5.48% Ni, 0.5 3% Co, 0.23% P, 61 ppm Ga, 170 ppm Ge, the main mass now weighs 16.3 kg and measures 22 x 15 x 43 ppm Ir. 13 em. A large end piece of 7 kg and several slices have been removed, leaving a cut surface of 17 x 10 em. The mass has HISTORY a relatively smooth domed surface (22 x 15 em) overlying a A mass was found at the coordinates given above, on concave surface with irregular depressions, from a few em the railway between Calama and Antofagasta, close to to 8 em in length. There is a series of what appears to be Sierra Gorda, the location of a silver mine (E.P. Henderson chisel marks around the center of the domed surface over 1939; as quoted by Hey 1966: 448). Henderson (1941a) an area of 6 x 7 em. Other small areas on the edges of the gave slightly different coordinates and an analysis; but since specimen could also be the result of hammering; but the he assumed Sierra Gorda to be just another of the North damage is only superficial, and artificial reheating has not Chilean hexahedrites, no further description was given. -

Laboratory Spectroscopy of Meteorite Samples at UV-Vis-NIR Wavelengths: Analysis and Discrimination by Principal Components Analysis
Laboratory spectroscopy of meteorite samples at UV-Vis-NIR wavelengths: Analysis and discrimination by principal components analysis Antti Penttil¨aa,∗, Julia Martikainena, Maria Gritsevicha, Karri Muinonena,b aDepartment of Physics, P.O. Box 64, FI-00014 University of Helsinki, Finland bFinnish Geospatial Research Institute FGI, National Land Survey of Finland, Geodeetinrinne 2, FI-02430 Masala, Finland Abstract Meteorite samples are measured with the University of Helsinki integrating-sphere UV-Vis- NIR spectrometer. The resulting spectra of 30 meteorites are compared with selected spectra from the NASA Planetary Data System meteorite spectra database. The spectral measure- ments are transformed with the principal component analysis, and it is shown that different meteorite types can be distinguished from the transformed data. The motivation is to im- prove the link between asteroid spectral observations and meteorite spectral measurements. Keywords: Meteorites, spectroscopy, principal component analysis 1. Introduction While a planet orbits the Sun, it is subject to impacts by objects ranging from tiny dust particles to much larger asteroids and comet nuclei. Such collisions of small Solar System bodies with planets have taken place frequently over geological time and played an 5 important role in the evolution of planets and development of life on the Earth. Every day approximately 30{180 tons of interplanetary material enter the Earth's atmosphere [1, 2]. This material is mostly represented by smaller meteoroids that undergo rapid ablation in the atmosphere. Under favorable initial conditions part of a meteoroid may survive the atmospheric entry and reach the ground [3]. The fragments recovered on the ground are 10 called meteorites, our valuable samples of the Solar System. -

Constraining the Source Regions of Lunar Meteorites Using Orbital Geochemical Data
Meteoritics & Planetary Science 50, Nr 2, 214–228 (2015) doi: 10.1111/maps.12412 Constraining the source regions of lunar meteorites using orbital geochemical data A. CALZADA-DIAZ1,2*, K. H. JOY3, I. A. CRAWFORD1,2, and T. A. NORDHEIM2,4 1Department of Earth and Planetary Sciences, Birkbeck College, London WC1E 7HX, UK 2Centre for Planetary Sciences UCL/Birkbeck, London WC1E 6BT, UK 3School of Earth, Atmospheric and Environmental Sciences, University of Manchester, Manchester M13 9PL, UK 4Mullard Space Science Laboratory, University College London, Dorking RH5 6NT, UK *Corresponding author. E-mail: [email protected] (Received 30 July 2014; revision accepted 06 November 2014) Abstract–Lunar meteorites provide important new samples of the Moon remote from regions visited by the Apollo and Luna sample return missions. Petrologic and geochemical analysis of these meteorites, combined with orbital remote sensing measurements, have enabled additional discoveries about the composition and age of the lunar surface on a global scale. However, the interpretation of these samples is limited by the fact that we do not know the source region of any individual lunar meteorite. Here, we investigate the link between meteorite and source region on the Moon using the Lunar Prospector gamma ray spectrometer remote sensing data set for the elements Fe, Ti, and Th. The approach has been validated using Apollo and Luna bulk regolith samples, and we have applied it to 48 meteorites excluding paired stones. Our approach is able broadly to differentiate the best compositional matches as potential regions of origin for the various classes of lunar meteorites. Basaltic and intermediate Fe regolith breccia meteorites are found to have the best constrained potential launch sites, with some impact breccias and pristine mare basalts also having reasonably well-defined potential source regions. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Discovery of Amino Acids from Didwana-Ra Jod
DISCUSSION DISCOVERY OF AMINO ACIDS FROM DIDWANA-RAJOD METEORITE AND ITS IMPLICATIONS ON ORIGIN OF LIFE by Vinod C. Tewari et al. Jour. Geol. SQC.India, v.60,2002, pp. 107-1 10. geochemistry of meteorite amino acids is scanty at present \ for any meaningful interpretation of isotope data. Therefore, P-IL Sukumaran, Geological Survey of Xrrdia, Alandi the presence of three a amino acids reported by the auaars Road, Pune - 411 OQ6, Ernail: [email protected];.o.uk, cannot be taken as conclusive evidence for their biogenicity, comments: more so in the absence of stereochemical and stable isotope data. The greatest mystery in science is the origin of life and Another point that calls for attention is the serious the greatest discovery in science will be the discovery of problem of contamination faced while studying meteoritic life beyond earth, if at all extt-aterrestriaF life would ever be organic compounds. The authors cIaim that their samples discovered. ft is in this context that I read with interest the are free of contamination without giving any details. Many research communication by Vinod C. Tewari et al. on the studies published earlier in the literature on meteorite discovery of amino acids in the Didwana-Rajod meteorite. organics have subsequently been rejected based on the fact However, there is little description of the meteorite, as to that they are all terrestrial contaminants. when did it fall, its repository, etc., as these aspects are Attention of the authors is also drawn to two papers that very important while studying the amino acids in the appeared in March 2002 issue of Nature. -

Did a Comet Deliver the Chelyabinsk Meteorite?
EPSC Abstracts Vol. 11, EPSC2017-2, 2017 European Planetary Science Congress 2017 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2017 Did a Comet Deliver the Chelyabinsk Meteorite? O.G. Gladysheva Ioffe Physical-Technical Institute of RAS, St.Petersburg, Russa, ([email protected] / Fax: +7(812)2971017) Abstract source of the appearance of such a gradient can be only the direct bombardment of the crystal by SCR An explosion of a celestial body occurred on the iron nuclei with energy of 1-100 MeV [6]. fifteenth of February, 2013, near Chelyabinsk Interacting with the surface of the meteorite, protons (Russia). The explosive energy was determined as and helium GCR nuclei form isotopes, some ~500 kt of TNT, on the basis of which the mass of radioactive, which are allocated to a specific location the bolide was estimated at ~107 kg, and its diameter by depth in the body of the meteorite. A measurement of the composition of radionuclides at ~19 m [1]. Fragments of the meteorite, such as 22 26 54 60 LL5/S4-WO type ordinary chondrite [2] with a total Na, Al, Mn and Co in 12 fragments of the mass only of ~2·103 kg, fell to the earth’s surface [3]. Chelyabinsk meteorite, and a comparison of the Here, we will demonstrate that the deficit of the results with model calculations of the formation of celestial body’s mass can be explained by the arrival these isotopes in meteorites according to depth, of the Chelyabinsk chondrite on Earth by a showed that 4 fragments of the meteorite were significantly more massive but fragile ice-bearing located in a layer 30 cm deep, 3 fragments at a depth celestial body. -

CHAPTER 1 Introduction
Chemical analysis of organic molecules in carbonaceous meteorites Torrao Pinto Martins, Zita Carla Citation Torrao Pinto Martins, Z. C. (2007, January 24). Chemical analysis of organic molecules in carbonaceous meteorites. Retrieved from https://hdl.handle.net/1887/9450 Version: Corrected Publisher’s Version Licence agreement concerning inclusion of doctoral License: thesis in the Institutional Repository of the University of Leiden Downloaded from: https://hdl.handle.net/1887/9450 Note: To cite this publication please use the final published version (if applicable). ______________________________________________________ CHAPTER 1 ______________________________________________________ Introduction 1.1 Heavenly stones-from myth to science Ancient chronicles, from the Egyptian, Chinese, Greek, Roman and Sumerian civilizations documented the fall1 of meteorites, with Sumerian texts from around the end of the third millennium B. C. describing possibly one of the earliest words for meteoritic iron (Fig. 1.1 Left). Egyptian hieroglyphs meaning “heavenly iron” (Fig. 1.1 Right) found in pyramids together with the use of meteoritic iron in jewellery and artefacts show the importance of meteorites in early Egypt. Meteorites were worshiped by ancient Greeks and Romans, who struck coins to celebrate their fall, with the cult to worship meteorites prevailing for many centuries. For example, some American Indian tribes paid tribute to large iron meteorites, and even in modern days the Black Stone of the Ka´bah in Mecca is worshiped and regarded by Muslims as “an object from heaven”. The oldest preserved meteorite that was observed to fall (19th May 861) was found recently (October 1979) in a Shinto temple in Nogata, Japan. It weighted 472 g and it was stored in a wooden box. -

Tree-Ring Dating of Meteorite Fall in Sikhote-Alin, Eastern Siberia – Russia
International Journal of Astrobiology, Page 1 of 6 1 doi:10.1017/S1473550411000309 © Cambridge University Press 2011 Tree-ring dating of meteorite fall in Sikhote-Alin, Eastern Siberia – Russia R. Fantucci1, Mario Di Martino2 and Romano Serra3 1Geologi Associati Fantucci e Stocchi, 01027 Montefiascone (VT), Italy e-mail: [email protected] 2INAF-Osservatorio Astronomico di Torino, 10025 Pino Torinese, Italy 3Dipartimento di Fisica, Università di Bologna, via Irnerio 46, 40126 Bologna, Italy Abstract: This research deals with the fall of the Sikhote-Alin iron meteorite on the morning of 12 February 1947, at about 00:38 h Utrecht, in a remote area in the territory of Primorsky Krai in Eastern Siberia (46°09′ 36″N, 134°39′22″E). The area engulfed by the meteoritic fall was around 48 km2, with an elliptic form and thousands of craters. Around the large craters the trees were torn out by the roots and laid radially to the craters at a distance of 10–20 m; the more distant trees had broken tops. This research investigated through dendrocronology n.6 Scots pine trees (Pinus Sibirica) close to one of the main impact craters. The analysis of growth anomalies has shown a sudden decrease since 1947 for 4–8 years after the meteoritic impact. Tree growth stress, detected in 1947, was analysed in detail through wood microsection that confirmed the winter season (rest vegetative period) of the event. The growth stress is mainly due to the lost crown (needle lost) and it did not seem to be caused due to direct damages on trunk and branches (missing of resin ducts). -
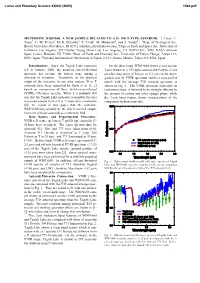
Meteorite Wis91600: a New Sample Related to a D- Or T-Type Asteroid
Lunar and Planetary Science XXXVI (2005) 1564.pdf METEORITE WIS91600: A NEW SAMPLE RELATED TO A D- OR T-TYPE ASTEROID. T. Hiroi1, E. Tonui2, C. M. Pieters1, M. E. Zolensky3, Y. Ueda4, M. Miyamoto4, and S. Sasaki4,5, 1Dept. of Geological Sci., Brown University, Providence, RI 02912 ([email protected]), 2Dept. of Earth and Space Sci., University of California Los Angeles, 595 Charles Young Drive East, Los Angeles, CA 90095-1567, 3SN2, NASA Johnson Space Center, Houston, TX 77058, 4Dept. of Earth and Planetary Sci., University of Tokyo, Hongo, Tokyo 113- 0033, Japan, 5National Astronomical Observatory of Japan, 2-21-1 Osawa, Mitaka, Tokyo 181-8588, Japan. Introduction: Since the Tagish Lake meteorite On the other hand, WIS91600 shows a very similar fell in January 2000, the assumed one-of-the-kind 3-µm feature to a T/D-type asteroid 308 Polyxo, if one meteorite has become the hottest issue among a peculiar data point of Polyxo at 3.5 µm can be disre- diversity of scientists. Meanwhile, as the physical garded and its VNIR spectrum shows a near-perfect origin of the meteorite in our solar system, D or T match with the average T/D asteroid spectrum as asteroids have been suggested by Hiroi et al. [1, 2] shown in Fig. 1. The VNIR spectrum, especially its based on comparison of their visible-near-infrared continuum slope, is believed to be strongly affected by (VNIR) reflectance spectra. While it is probably still the amount of carbon and other opaque phase, while true that the Tagish Lake meteorite is possibly the first the 3-µm band feature shows characteristics of the recovered sample from a D or T asteroid as a meteorite component hydrous minerals. -

Radar-Enabled Recovery of the Sutter's Mill Meteorite, A
RESEARCH ARTICLES the area (2). One meteorite fell at Sutter’sMill (SM), the gold discovery site that initiated the California Gold Rush. Two months after the fall, Radar-Enabled Recovery of the Sutter’s SM find numbers were assigned to the 77 me- teorites listed in table S3 (3), with a total mass of 943 g. The biggest meteorite is 205 g. Mill Meteorite, a Carbonaceous This is a tiny fraction of the pre-atmospheric mass, based on the kinetic energy derived from Chondrite Regolith Breccia infrasound records. Eyewitnesses reported hearing aloudboomfollowedbyadeeprumble.Infra- Peter Jenniskens,1,2* Marc D. Fries,3 Qing-Zhu Yin,4 Michael Zolensky,5 Alexander N. Krot,6 sound signals (table S2A) at stations I57US and 2 2 7 8 8,9 Scott A. Sandford, Derek Sears, Robert Beauford, Denton S. Ebel, Jon M. Friedrich, I56US of the International Monitoring System 6 4 4 10 Kazuhide Nagashima, Josh Wimpenny, Akane Yamakawa, Kunihiko Nishiizumi, (4), located ~770 and ~1080 km from the source, 11 12 10 13 Yasunori Hamajima, Marc W. Caffee, Kees C. Welten, Matthias Laubenstein, are consistent with stratospherically ducted ar- 14,15 14 14,15 16 Andrew M. Davis, Steven B. Simon, Philipp R. Heck, Edward D. Young, rivals (5). The combined average periods of all 17 18 18 19 20 Issaku E. Kohl, Mark H. Thiemens, Morgan H. Nunn, Takashi Mikouchi, Kenji Hagiya, phase-aligned stacked waveforms at each station 21 22 22 22 23 Kazumasa Ohsumi, Thomas A. Cahill, Jonathan A. Lawton, David Barnes, Andrew Steele, of 7.6 s correspond to a mean source energy of 24 4 24 2 25 Pierre Rochette, Kenneth L. -

Calcium Isotopes in Natural and Experimental Carbonated Silicate Melts
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 2-27-2018 2:30 PM Calcium Isotopes in Natural and Experimental Carbonated Silicate Melts Matthew Maloney The University of Western Ontario Supervisor Bouvier, Audrey The University of Western Ontario Co-Supervisor Withers, Tony The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Matthew Maloney 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Geochemistry Commons Recommended Citation Maloney, Matthew, "Calcium Isotopes in Natural and Experimental Carbonated Silicate Melts" (2018). Electronic Thesis and Dissertation Repository. 5256. https://ir.lib.uwo.ca/etd/5256 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract The calcium stable isotopic compositions of mantle-sourced rocks and minerals were investigated to better understand the carbon cycle in the Earth’s mantle. Bulk carbonatites and kimberlites were analyzed to identify a geochemical signature of carbonatite magmatism, while inter-mineral fractionation was measured in co-existing Ca-bearing carbonate and silicate minerals. Bulk samples show a range of composition deviating from the bulk silicate Earth δ44/40Ca composition indicating signatures of magmatic processes or marine carbonate addition 44/40 to source materials. Δ Cacarbonate-silicate values range from -0.55‰ to +1.82‰ and positively correlate with Ca/Mg ratios in pyroxenes. -

Asteroid Regolith Weathering: a Large-Scale Observational Investigation
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2019 Asteroid Regolith Weathering: A Large-Scale Observational Investigation Eric Michael MacLennan University of Tennessee, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation MacLennan, Eric Michael, "Asteroid Regolith Weathering: A Large-Scale Observational Investigation. " PhD diss., University of Tennessee, 2019. https://trace.tennessee.edu/utk_graddiss/5467 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Eric Michael MacLennan entitled "Asteroid Regolith Weathering: A Large-Scale Observational Investigation." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Geology. Joshua P. Emery, Major Professor We have read this dissertation and recommend its acceptance: Jeffrey E. Moersch, Harry Y. McSween Jr., Liem T. Tran Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Asteroid Regolith Weathering: A Large-Scale Observational Investigation A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Eric Michael MacLennan May 2019 © by Eric Michael MacLennan, 2019 All Rights Reserved.