Soluble Organic Compounds in the Tagish Lake Meteorite. R.W. Hilts1 and C.D.K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constraining the Source Regions of Lunar Meteorites Using Orbital Geochemical Data
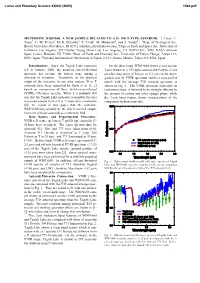
Meteoritics & Planetary Science 50, Nr 2, 214–228 (2015) doi: 10.1111/maps.12412 Constraining the source regions of lunar meteorites using orbital geochemical data A. CALZADA-DIAZ1,2*, K. H. JOY3, I. A. CRAWFORD1,2, and T. A. NORDHEIM2,4 1Department of Earth and Planetary Sciences, Birkbeck College, London WC1E 7HX, UK 2Centre for Planetary Sciences UCL/Birkbeck, London WC1E 6BT, UK 3School of Earth, Atmospheric and Environmental Sciences, University of Manchester, Manchester M13 9PL, UK 4Mullard Space Science Laboratory, University College London, Dorking RH5 6NT, UK *Corresponding author. E-mail: [email protected] (Received 30 July 2014; revision accepted 06 November 2014) Abstract–Lunar meteorites provide important new samples of the Moon remote from regions visited by the Apollo and Luna sample return missions. Petrologic and geochemical analysis of these meteorites, combined with orbital remote sensing measurements, have enabled additional discoveries about the composition and age of the lunar surface on a global scale. However, the interpretation of these samples is limited by the fact that we do not know the source region of any individual lunar meteorite. Here, we investigate the link between meteorite and source region on the Moon using the Lunar Prospector gamma ray spectrometer remote sensing data set for the elements Fe, Ti, and Th. The approach has been validated using Apollo and Luna bulk regolith samples, and we have applied it to 48 meteorites excluding paired stones. Our approach is able broadly to differentiate the best compositional matches as potential regions of origin for the various classes of lunar meteorites. Basaltic and intermediate Fe regolith breccia meteorites are found to have the best constrained potential launch sites, with some impact breccias and pristine mare basalts also having reasonably well-defined potential source regions. -
Meteorite Wis91600: a New Sample Related to a D- Or T-Type Asteroid
Lunar and Planetary Science XXXVI (2005) 1564.pdf METEORITE WIS91600: A NEW SAMPLE RELATED TO A D- OR T-TYPE ASTEROID. T. Hiroi1, E. Tonui2, C. M. Pieters1, M. E. Zolensky3, Y. Ueda4, M. Miyamoto4, and S. Sasaki4,5, 1Dept. of Geological Sci., Brown University, Providence, RI 02912 ([email protected]), 2Dept. of Earth and Space Sci., University of California Los Angeles, 595 Charles Young Drive East, Los Angeles, CA 90095-1567, 3SN2, NASA Johnson Space Center, Houston, TX 77058, 4Dept. of Earth and Planetary Sci., University of Tokyo, Hongo, Tokyo 113- 0033, Japan, 5National Astronomical Observatory of Japan, 2-21-1 Osawa, Mitaka, Tokyo 181-8588, Japan. Introduction: Since the Tagish Lake meteorite On the other hand, WIS91600 shows a very similar fell in January 2000, the assumed one-of-the-kind 3-µm feature to a T/D-type asteroid 308 Polyxo, if one meteorite has become the hottest issue among a peculiar data point of Polyxo at 3.5 µm can be disre- diversity of scientists. Meanwhile, as the physical garded and its VNIR spectrum shows a near-perfect origin of the meteorite in our solar system, D or T match with the average T/D asteroid spectrum as asteroids have been suggested by Hiroi et al. [1, 2] shown in Fig. 1. The VNIR spectrum, especially its based on comparison of their visible-near-infrared continuum slope, is believed to be strongly affected by (VNIR) reflectance spectra. While it is probably still the amount of carbon and other opaque phase, while true that the Tagish Lake meteorite is possibly the first the 3-µm band feature shows characteristics of the recovered sample from a D or T asteroid as a meteorite component hydrous minerals. -

Chelyabinsk Airburst, Damage Assessment, Meteorite Recovery and Characterization
O. P. Popova, et al., Chelyabinsk Airburst, Damage Assessment, Meteorite Recovery and Characterization. Science 342 (2013). Chelyabinsk Airburst, Damage Assessment, Meteorite Recovery, and Characterization Olga P. Popova1, Peter Jenniskens2,3,*, Vacheslav Emel'yanenko4, Anna Kartashova4, Eugeny Biryukov5, Sergey Khaibrakhmanov6, Valery Shuvalov1, Yurij Rybnov1, Alexandr Dudorov6, Victor I. Grokhovsky7, Dmitry D. Badyukov8, Qing-Zhu Yin9, Peter S. Gural2, Jim Albers2, Mikael Granvik10, Läslo G. Evers11,12, Jacob Kuiper11, Vladimir Kharlamov1, Andrey Solovyov13, Yuri S. Rusakov14, Stanislav Korotkiy15, Ilya Serdyuk16, Alexander V. Korochantsev8, Michail Yu. Larionov7, Dmitry Glazachev1, Alexander E. Mayer6, Galen Gisler17, Sergei V. Gladkovsky18, Josh Wimpenny9, Matthew E. Sanborn9, Akane Yamakawa9, Kenneth L. Verosub9, Douglas J. Rowland19, Sarah Roeske9, Nicholas W. Botto9, Jon M. Friedrich20,21, Michael E. Zolensky22, Loan Le23,22, Daniel Ross23,22, Karen Ziegler24, Tomoki Nakamura25, Insu Ahn25, Jong Ik Lee26, Qin Zhou27, 28, Xian-Hua Li28, Qiu-Li Li28, Yu Liu28, Guo-Qiang Tang28, Takahiro Hiroi29, Derek Sears3, Ilya A. Weinstein7, Alexander S. Vokhmintsev7, Alexei V. Ishchenko7, Phillipe Schmitt-Kopplin30,31, Norbert Hertkorn30, Keisuke Nagao32, Makiko K. Haba32, Mutsumi Komatsu33, and Takashi Mikouchi34 (The Chelyabinsk Airburst Consortium). 1Institute for Dynamics of Geospheres of the Russian Academy of Sciences, Leninsky Prospect 38, Building 1, Moscow, 119334, Russia. 2SETI Institute, 189 Bernardo Avenue, Mountain View, CA 94043, USA. 3NASA Ames Research Center, Moffett Field, Mail Stop 245-1, CA 94035, USA. 4Institute of Astronomy of the Russian Academy of Sciences, Pyatnitskaya 48, Moscow, 119017, Russia. 5Department of Theoretical Mechanics, South Ural State University, Lenin Avenue 76, Chelyabinsk, 454080, Russia. 6Chelyabinsk State University, Bratyev Kashirinyh Street 129, Chelyabinsk, 454001, Russia. -

N Arieuican%Mllsellm
n ARieuican%Mllsellm PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2I63 DECEMBER I9, I963 The Pallasites BY BRIAN MASON' INTRODUCTION The pallasites are a comparatively rare type of meteorite, but are remarkable in several respects. Historically, it was a pallasite for which an extraterrestrial origin was first postulated because of its unique compositional and structural features. The Krasnoyarsk pallasite was discovered in 1749 about 150 miles south of Krasnoyarsk, and seen by P. S. Pallas in 1772, who recognized these unique features and arranged for its removal to the Academy of Sciences in St. Petersburg. Chladni (1794) examined it and concluded it must have come from beyond the earth, at a time when the scientific community did not accept the reality of stones falling from the sky. Compositionally, the combination of olivine and nickel-iron in subequal amounts clearly distinguishes the pallasites from all other groups of meteorites, and the remarkable juxtaposition of a comparatively light silicate mineral and heavy metal poses a nice problem of origin. Several theories of the internal structure of the earth have postulated the presence of a pallasitic layer to account for the geophysical data. No apology is therefore required for an attempt to provide a comprehensive account of this remarkable group of meteorites. Some 40 pallasites are known, of which only two, Marjalahti and Zaisho, were seen to fall (table 1). Of these, some may be portions of a single meteorite. It has been suggested that the pallasite found in Indian mounds at Anderson, Ohio, may be fragments of the Brenham meteorite, I Chairman, Department of Mineralogy, the American Museum of Natural History. -

Trace Element Chemistry of Cumulus Ridge 04071 Pallasite with Implications for Main Group Pallasites
Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Item Type Article; text Authors Danielson, L. R.; Righter, K.; Humayun, M. Citation Danielson, L. R., Righter, K., & Humayun, M. (2009). Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites. Meteoritics & Planetary Science, 44(7), 1019-1032. DOI 10.1111/j.1945-5100.2009.tb00785.x Publisher The Meteoritical Society Journal Meteoritics & Planetary Science Rights Copyright © The Meteoritical Society Download date 23/09/2021 14:17:54 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/656592 Meteoritics & Planetary Science 44, Nr 7, 1019–1032 (2009) Abstract available online at http://meteoritics.org Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Lisa R. DANIELSON1*, Kevin RIGHTER2, and Munir HUMAYUN3 1Mailcode JE23, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 2Mailcode KT, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 3National High Magnetic Field Laboratory and Department of Geological Sciences, Florida State University, Tallahassee, Florida 32310, USA *Corresponding author. E-mail: [email protected] (Received 06 November 2008; revision accepted 11 May 2009) Abstract–Pallasites have long been thought to represent samples from the metallic core–silicate mantle boundary of a small asteroid-sized body, with as many as ten different parent bodies recognized recently. This report focuses on the description, classification, and petrogenetic history of pallasite Cumulus Ridge (CMS) 04071 using electron microscopy and laser ablation ICP-MS. -

Chondrules and Their Origins. Subject Index
359 1983chto.conf..359. SUBJECT INDEX Ablation spherules 21 formation 46, 50 as chondrule analogs 11, 22 internal isochrons 251, 256 composition 14, 15, 17, 18 iodine-xenon dating 256 formation 11-14, 19 matrix ages 257 grains 13, 14 metamorphic structure 47 iron 14 origin 50 magnetic properties 15 photomicrographs 47 matrix 13 potassium-argon dating 251 melting 19, 21, 22 texture 44 metals 19 ultrathin sections 47 mineralogy 19 Allende chondrules photomicrographs 13 ages 247-251,253,255-257 porphyritic 21 alteration 159 siderophiles 15-19 cavities 155-157, 159 texture 19 classification 207 thermal effects 12-14 composition 42, 93, 156, 157 volatiles 13, 14 EPMA 93 Aerodynamic spherules extraction temperature 254 as chondrule analogs 10, 12 formation 160 composition 12 iodine 254, 256, 257 formation 12 iodine-xenon 258 thermal effects 12 isochrons 254 Age dating techniques 257 isotopes 37, 39-42, 270 iodine-xenon 246, 247 mineralogy 93, 149-152, 154, 155, 158, 160 isochrons 257 mixing line 41 metamorphic processes 257 opaque inclusions 155, 159 potassium-argon 246, 247 photomicrographs 148, 150, 151, 154, 156, rubidium-strontium 246, 247 157 uranium-lead 246, 247 precursors 42 Ages of chondrules sample analysis 39 iodine-xenon 246, 247, 251-257 SEM studies 150, 151, 152, 154, 156, 157 potassium-argon 246, 247, 250, 251, 257, 258 texture 39, 41, 155 rubidium-strontium 246-250, 258 thermal history 255 uranium-lead 246-248, 258 xenon closure 254 Allegan Allende inclusions 145 bulk analysis of chondrules 48, 49 ages 255 chemistry 46 initial iodine 256· chondrule ages 251, 256, 257 iodine-xenon dating 255 chondrule oxygen isotope analysis 40 isochrons 255 chondrules 44, 47 isotope analysis 39 classification 46, 50 sample analysis 39 composition 46 texture 39 description 46 Allende matrix 145, 257 equilibration 44, 50 alteration 159 © Lunar and Planetary Institute • Provided by the NASA Astrophysics Data System 360 Chondrules and their Origins 1983chto.conf..359. -

Proceedings of the International Meteor Conference La Palma, Canary Islands, Spain 20–23 September, 2012
ISBN 978-2-87355-024-4 Proceedings of the International Meteor Conference La Palma, Canary Islands, Spain 20–23 September, 2012 Published by the International Meteor Organization 2013 Edited by Marc Gyssens and Paul Roggemans Proceedings of the International Meteor Conference La Palma, Canary Islands, Spain, 20–23 September, 2012 International Meteor Organization ISBN 978-2-87355-024-4 Copyright notices c 2013 The International Meteor Organization The copyright of papers in this publication remains with the authors. It is the aim of the IMO to increase the spread of scientific information, not to restrict it. When material is submitted to the IMO for publication, this is taken as indicating that the author(s) grant(s) permission for the IMO to publish this material any number of times, in any format(s), without payment. This permission is taken as covering rights to reproduce both the content of the material and its form and appearance, including images and typesetting. Formats may include paper and electronically readable storage media. Other than these conditions, all rights remain with the author(s). When material is submitted for publication, this is also taken as indicating that the author(s) claim(s) the right to grant the permissions described above. The reader is granted permission to make unaltered copies of any part of the document for personal use, as well as for non-commercial and unpaid sharing of the information with third parties, provided the source and publisher are mentioned. For any other type of copying or distribution, prior written permission from the publisher is mandatory. -

Spectral Characteristics of Ordinary Chondrite Impact Melts
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 1594.pdf SPECTRAL CHARACTERISTICS OF ORDINARY CHONDRITE IMPACT MELTS. J. A. Sanchez1, V. Reddy2, L. Le Corre1, T. Campbell2, O. Chabra3, 1Planetary Science Institute, 1700 East Fort Lowell, Suite 106, Tuc- son, AZ 85719-2395, 2Lunar and Planetary Laboratory, University of Arizona, 1629 E University Blvd, Tucson, AZ 85721-0092, 3Catalina Foothills High School, 4300 E Sunrise Dr, Tucson, AZ 85718 Introduction: Impacts are the most ubiquitous of all the processes that shape solar system bodies. Evidence for these collisions is visible on a macro scale in the form of impact craters and collisional fragments (i.e. aster- oids). Impacts on larger bodies such as terrestrial plan- ets and our own Moon modify the initial target material texture and composition creating impact melts that can be detected from remote sensing and also in returned samples. The production of impact melt is a function of impact velocity. Most asteroidal collisions take place at velocities of ~5 km/sec [1], which is sufficient to pro- duce impact melt deposits as seen on the Moon where the impact velocities are much higher (~15 km/sec). Figure 1: Sample of Chelyabinsk LL5 chondrite. Half However, porosity and composition (presence of of the sample is formed by a light (unaltered) lithology metal/metal sulfides) play an equally important role as while the other half by shock-blackened/impact melt impact velocity [2]. material. Several meteorites derived from asteroidal sources show evidence for shock-darkening and impact Results: Figures 2 to 4 show the NIR spectra of the melt. -

Black As Coal . Hard As Rock. Ordinary As a Chondrite
Meteorite-Times Magazine Contents by Editor Like Sign Up to see what your friends like. Featured Monthly Articles Accretion Desk by Martin Horejsi Jim’s Fragments by Jim Tobin Meteorite Market Trends by Michael Blood Bob’s Findings by Robert Verish IMCA Insights by The IMCA Team Micro Visions by John Kashuba Galactic Lore by Mike Gilmer Meteorite Calendar by Anne Black Meteorite of the Month by Michael Johnson Tektite of the Month by Editor Terms Of Use Materials contained in and linked to from this website do not necessarily reflect the views or opinions of The Meteorite Exchange, Inc., nor those of any person connected therewith. In no event shall The Meteorite Exchange, Inc. be responsible for, nor liable for, exposure to any such material in any form by any person or persons, whether written, graphic, audio or otherwise, presented on this or by any other website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. does not endorse, edit nor hold any copyright interest in any material found on any website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. shall not be held liable for any misinformation by any author, dealer and or seller. In no event will The Meteorite Exchange, Inc. be liable for any damages, including any loss of profits, lost savings, or any other commercial damage, including but not limited to special, consequential, or other damages arising out of this service. © Copyright 2002–2010 The Meteorite Exchange, Inc. All rights reserved. No reproduction of copyrighted material is allowed by any means without prior written permission of the copyright owner. -

Late Accretion and Lithification of Chondritic Parent Bodies: Mg Isotope Studies on Fragments from Primitive Chondrites and Chondritic Breccias
Meteoritics & Planetary Science 42, Nr 7/8, 1291–1308 (2007) Abstract available online at http://meteoritics.org Late accretion and lithification of chondritic parent bodies: Mg isotope studies on fragments from primitive chondrites and chondritic breccias Anna K. SOKOL1, 3*, Addi BISCHOFF1, Kuljeet K. MARHAS2, Klaus MEZGER3, and Ernst ZINNER2 1Institut für Planetologie, Wilhelm-Klemm-Str. 10, 48149 Münster, Germany 2McDonnell Center for the Space Sciences and Physics Department, Washington University, One Brookings Drive, Saint Louis, Missouri 63130, USA 3Institut für Mineralogie, Corrensstr. 24, 48149 Münster, Germany *Corresponding author. E-mail: [email protected] (Received 16 October 2006; revision accepted 25 July 2007) Abstract–Recent results of isotopic dating studies (182Hf-182W, 26Al-26Mg) and the increasing number of observed igneous and metamorphosed fragments in (primitive) chondrites provide strong evidence that accretion of differentiated planetesimals predates that of primitive chondrite parent bodies. The primitive chondrites Adrar 003 and Acfer 094 contain some unusual fragments that seem to have undergone recrystallization. Magnesium isotope analyses reveal no detectable radiogenic 26Mg in any of the studied fragments. The possibility that evidence for 26Al was destroyed by parent body metamorphism after formation is not likely because several other constituents of these chondrites do not show any metamorphic features. Since final accretion of a planetesimal must have occurred after formation of its youngest components, formation of these parent bodies must thus have been relatively late (i.e., after most 26Al had decayed). Al-Mg isotope data for some igneous-textured clasts (granitoids and andesitic fragments) within the two chondrite regolith breccias Adzhi-Bogdo and Study Butte reveal also no evidence for radiogenic 26Mg. -

The Kaidun Meteorite
Lunar and Planetary Science XXXIV (2003) 1236.pdf THE KAIDUN METEORITE: WHERE DID IT COME FROM? Andrei Ivanov1 and Michael Zolensky2; 1Vernadsky Institute, 119991 Moscow, Russia; 2NASA Johnson Space Center, Houston, TX 77058, USA. The Kaidun meteorite, which fell on The alkaline-enriched rocks are 3.12.1980 at lat. 15° N, long. 48.3° E, holds a represented by two different fragments. One special place in the world meteorite collection. is a fragment of a twinned albite crystal 1.2 x Kaidun is characterized by an unprecedentedly 0.7 mm in size, with inclusions of fluorapatite, wide variety of meteorite material in its aenigmatite, wilkinsonite and arfvedsonite makeup. The high degree of variability in this [15]. Importantly arfvedsonite, aenigmatite meteorite’s material is evidenced by the and wilkinsonite are represented by their richness of its mineral composition – nearly 60 magnesium-rich forms, and the latter two minerals and mineral phases have been minerals in this parameter and also in the identified in Kaidun, including several never relatively low content of manganese before found in nature, such as florenskiite noticeably differ from terrestrial examples. FeTiP, the first known phosphide of a The second fragment is a melted clast ~3 lithophilic element [1]. mm in size [16]. The relationship of this clast to the rock which contains it indicates the clast Lithologic Composition of the Meteorite was melted in situ. The matrix of the clast Kaidun’s matrix is made up of CR2 consists of tabletlike, skeletal and boxy carbonaceous chondrite material, identified plagioclase crystals ( average composition through mineralogical, petrographic, chemical An22Ab77) in a glassy mass. -

The Meteoric Ablation Simulator (MASI) D
A novel instrument to measure differential ablation of meteorite samples and proxies: The Meteoric Ablation Simulator (MASI) D. L. Bones, J. C. Gómez Martín, C. J. Empson, J. D. Carrillo Sánchez, A. D. James, T. P. Conroy, and J. M. C. Plane Citation: Review of Scientific Instruments 87, 094504 (2016); doi: 10.1063/1.4962751 View online: http://dx.doi.org/10.1063/1.4962751 View Table of Contents: http://scitation.aip.org/content/aip/journal/rsi/87/9?ver=pdfcov Published by the AIP Publishing Articles you may be interested in Stardusts in Meteorites —Precursors of Planets AIP Conf. Proc. 847, 319 (2006); 10.1063/1.2234419 Supernova grains from meteorites AIP Conf. Proc. 402, 287 (1997); 10.1063/1.53330 Presolar material in meteorites: an overview AIP Conf. Proc. 402, 3 (1997); 10.1063/1.53326 Presolar oxide grains in meteorites AIP Conf. Proc. 402, 59 (1997); 10.1063/1.53320 Dust production in the galaxy: The meteorite perspective AIP Conf. Proc. 402, 567 (1997); 10.1063/1.53319 Reuse of AIP Publishing content is subject to the terms at: https://publishing.aip.org/authors/rights-and-permissions. Download to IP: 86.181.152.70 On: Wed, 28 Sep 2016 07:01:10 REVIEW OF SCIENTIFIC INSTRUMENTS 87, 094504 (2016) A novel instrument to measure differential ablation of meteorite samples and proxies: The Meteoric Ablation Simulator (MASI) D. L. Bones, J. C. Gómez Martín, C. J. Empson, J. D. Carrillo Sánchez, A. D. James, T. P. Conroy, and J. M. C. Planea) School of Chemistry, University of Leeds, Woodhouse Lane, LS2 9JT Leeds, United Kingdom (Received 9 June 2016; accepted 27 August 2016; published online 27 September 2016) On entering the Earth’s atmosphere, micrometeoroids partially or completely ablate, leaving behind layers of metallic atoms and ions.