Hemispheric Asymmetries of Cortical Volume in the Human Brain, Cortex (2011), Doi:10.1016/J.Cortex.2011.11.002 2 Cortex Xxx (2011) 1E11
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.EPISTEMOLOGICAL PERSPECTIVES in THE
Acta Colombiana de Psicología ISSN: 0123-9155 [email protected] Universidad Católica de Colombia Colombia Armengol de la Miyar, Carmen G.; Moes, Elisabeth J. EPISTEMOLOGICAL PERSPECTIVES IN THE SCIENTIFIC STUDY AND EVALUATION OF EXECUTIVE FUNCTION Acta Colombiana de Psicología, vol. 17, núm. 2, 2014, pp. 69-79 Universidad Católica de Colombia Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=79832492008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Acta.colomb.psicol. 17 (2): 69-79, 2014 http://www.dx.doi.org/10.14718/ACP.2014.17.2.8 EPISTEMOLOGICAL PERSPECTIVES IN THE SCIENTIFIC STUDY AND EVALUATION OF EXECUTIVE FUNCTION Dr. Carmen G. Armengol de la Miyar1*, Dr. Elisabeth J. Moes2** 1Counseling and Applied Psychology Department, Bouve College of Health Sciences, Northeastern University, Boston, Massachusetts, U.S.A. 2Department of Psychology, College of Arts and Sciences, Suffolk University, Boston, Massachusetts, U.S.A. Recibido, abril 25/2014 Referencia: Armengol de la Miyar, C.G. & Moes, E.J. Concepto de evaluación, mayo 12/2014 (2014). Epistemological perspectives in the scientific Aceptado, mayo 28/2014 study and clinical evaluation of executive function. Acta Colombiana de Psicología, 17 (2), pp. 69-79. DOI:10.14718/ ACP.2014.17.2.8 Abstract In this article, epistemological perspectives that have shaped and affected the scientific quest for understanding what neuropsychologists term “executive functions” are reviewed. Executive functions refer to the control functions of cognition and behavior. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Lateralization of Gene Expression at the Frontal Pole of the Human Brain Irina A
Psychology in Russia: State of the Art Russian Lomonosov Psychological Moscow State Volume 10, Issue 3, 2017 Society University Exploring terra incognita of cognitive science: Lateralization of gene expression at the frontal pole of the human brain Irina A. Dolinaa, Olga I. Efimovaa,b, Evgeniy M. Kildyushovc, Aleksey S. Sokolovd, Philipp E. Khaitovichb,e,f, ArtemV. Nedoluzhkoa, Fyodor S. Sharkoa, Boris M. Velichkovskya,h,i* a National Research Center “Kurchatov Institute”, Moscow, Russia b Skolkovo Institute for Science and Technology, Skolkovo, Russia c Pirogov Russian National Research Medical University, Moscow, Russia d Limited Liability Company “Elgene”, Krasnogorsk, Russia e CAS-MPG Partner Institute for Computational Biology, Shanghai, China f Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany h Moscow Institute for Physics and Technology, Moscow, Russia i Russian State University for the Humanities, Moscow, Russia * Corresponding author. E-mail: [email protected] Background. Rostral prefrontal cortex, or frontopolar cortex (FPC), also known as Brodmann area 10 (BA10), is the most anterior part of the human brain. It is one of the largest cytoarchitectonic areas of the human brain that has significantly increased its volume during evolution. Anatomically the left (BA10L) and right (BA10R) parts of FPC show slight asymmetries and they may have distinctive cognitive functions. Objective. In the present study, we investigated differential expression of the transcriptome in the left and right parts of BA10. Design. Postmortem samples of human brain tissue from fourteen donors (male/ female without history of psychiatric and neurological diseases, mean age 39.79±3.23 years old, mean postmortem interval 12.10±1.76 h) were obtained using the resources of three institutions: the Partner Institute of Computational Biology of Chinese Academy of Sciences, the Max Planck Institute for Evolutionary Anthropology, and NIH Neuro- BioBank. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

Resultado Final - Ordenado Por Departamento Item Autor Titulo Editora Q Preco Ptotal Depart
UNIVERSIDADE FEDERAL DE SÃO JOÃO DEL-REI EDITAL 001/2009 - RESULTADO FINAL - ORDENADO POR DEPARTAMENTO ITEM AUTOR TITULO EDITORA Q PRECO PTOTAL DEPART. PROFESSOR 1 DUNSTER, David 100 casas unifamiliares de la arquitectura del siglo XX Gustavo Gilli 2 100,97 201,94 Arquitetura Ana Cristina Reis Faria 2 CHOAY, Françoise A alegoria do patrimônio Estação Liberdade 5 48,00 240,00 Arquitetura Ana Cristina Reis Faria 3 ROMERO, Marta Adriana BuA arquitetura bioclimática do espaço público. UnB 5 32,00 160,00 Arquitetura Ana Cristina Reis Faria 4 WOLFFLIN, Heinrich A arte clássica Martins Fontes 5 56,00 280,00 Arquitetura Ana Cristina Reis Faria 5 WORRINGER, Wi A arte gótica. Edições 70 5 41,00 205,00 Arquitetura Ana Cristina Reis Faria 6 ABALOS, Iñaki. A boa-vida: visita guiada às casas da modernidade G. Gili, 5 84,00 420,00 Arquitetura Ana Cristina Reis Faria 7 RYKWERT, Joseph A casa de Adão no paraíso Perspectiva 5 47,00 235,00 Arquitetura Ana Cristina Reis Faria 8 BRANDÃO, Ludmila de LimA casa subjetiva. Matérias, afectos e espaços domésticos Perspectiva 5 40,00 200,00 Arquitetura Ana Cristina Reis Faria 9 CARLOS, Ana Fani A. A cidade Contexto 5 32,89 164,45 Arquitetura Ana Cristina Reis Faria 10 ARANTES, Otília Beatriz FioA cidade do pensamento único: desmanchando consensos Vozes 5 37,60 188,00 Arquitetura Ana Cristina Reis Faria 11 ROLNIK, Raquel. A cidade e a lei: legislação, política urbana e territórios na Studio Nobel 5 53,00 265,00 Arquitetura Ana Cristina Reis Faria 12 BENEVOLO, Leonardo A cidade e o arquiteto: método e historia na arquitetura. -

Webinar by Elkhonon Goldberg, Phd
Webinar by Elkhonon Goldberg, PhD WEBINAR “COVID-19 AND BRAIN DYSFUNCTION: EVOLVING UNDERSTANDING” COVID-19 is a viral illness caused by the novel coronavirus (SARS-CoV-2), which has become a global pandemic affecting all of us. While it has been originally characterized as respiratory illness, a growing body of evidence suggests that the brain may also be affected. In this webinar we will discuss the concept of “neuro-COVID” and examine the emerging evidence of COVID-19 impact on the human brain and the multiple clinical neurological and neuropsychological manifestations of this impact. In particular, we will discuss the potential for long-term neurocognitive sequelae of neuro-COVID and the role of neuropsychology in addressing them. In addition, we will briefly review the impact of diseases caused by other coronaviruses (SARS, MERS) on the brain. Date and time: December 10, 2020 (Thursday) from 2pm to 5:15pm Eastern Time (1pm – 4:15pm Central Time, 11am – 2:15pm Pacific Time) December 12, 2020 (Saturday) from 12pm to 3:15pm Eastern Time (11am – 2:15pm Central Time, 9am – 12:15pm Pacific Time) Topics to be covered: COVID-19 pandemic and the brain. Brain as the target of COVID-19. Direct vs indirect mechanisms of brain damage in COVID-19. Primary mechanisms of brain infection: transsynaptic vs hematogenous. Mechanisms of infection: the role of ACE2 receptor. COVID-19 and immune response. Clinical neurological and neuropsychiatric manifestations of COVID-19. Introducing “Neuro-COVID”. Long-term sequelae of Neuro-COVID. Other coronaviruses and the brain: SARS, MERS. Other viruses and the brain: HIV, and HSV. -
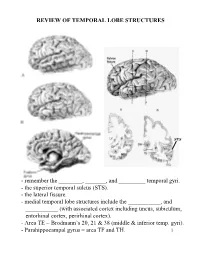
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

The Wisdom Paradox: How Your Mind Can Grow Stronger As Your Brain Grows Older
Wisdom and the Second Half of Life Books discussed: The Wisdom Paradox: How Your Mind Can Grow Stronger as Your Brain Grows Older. By Elkhonon Goldberg. New York: Gotham Books, 2005. viii + 336. $26 (cloth), $15 (paper) The Mature Mind: The Positive Power of the Aging Brain. By Gene Cohen. New York: Basic Books, 2005. xxiii + 232 pp. $24.95 (cloth), $15.95 (paper). I've always been absent-minded. From the beginning of grammar school I received demerits on my report card for daydreaming. When my eldest child was twelve or so, he began to complain about what he called my “dimwit autopilot.” The younger two children seized the label with great glee, and I’ve never lived it down. Nonetheless, I find myself worrying these days when I forget something, when I lose something, when I walk down to the basement and then wonder, "what was I after?" These days, when my mind wanders off, I worry: will it come back? Or am I losing it altogether? As an anxiously aging Boomer, then, I’ve been doing what I do best in any crisis: drinking tea and reading books. I’m pleased to report that my neurotic concerns are in fact plainly neurotic: research in the last ten or fifteen years has dramatically challenged the commonplace popular view of the aging process and especially the aging brain. The implications for our lives are both complex and profound. The major new fact is this: quite contrary to what was thought even ten years ago, the brain continues to grow new cells and to develop new physical capacities across the entire life span. -

Cerebral Asymmetry: a Quantitative, Multifactorial, and Plastic Brain Phenotype
Twin Research and Human Genetics Volume 15 Number 3 pp. 401–413 C The Authors 2012 doi:10.1017/thg.2012.13 Cerebral Asymmetry: A Quantitative, Multifactorial, and Plastic Brain Phenotype Miguel E. Renterıa´ 1,2 1Genetic Epidemiology and Quantitative Genetics laboratories, Queensland Institute of Medical Research, Brisbane, Australia 2School of Psychology, University of Queensland, Brisbane, Australia The longitudinal fissure separates the human brain into two hemispheres that remain connected through the corpus callosum. The left and the right halves of the brain resemble each other, and almost every structure present in one side has an equivalent structure in the other. Despite this exceptional correspondence, the two hemispheres also display important anatomical differences and there is marked lateralization of certain cognitive and motor functions such as language and handedness. However, the mechanisms that underlie the establishment of these hemispheric specializations, as well as their physiological and behavioral implications, remain largely unknown. Thanks to recent advances in neuroimaging, a series of studies documenting variation in symmetry and asymmetry as a function of age, gender, brain region, and pathological state, have been published in the past decade. Here, we review evidence of normal and atypical cerebral asymmetry, and the factors that influence it at the macrostructural level. Given the prominent role that cerebral asymmetry plays in the organization of the brain, and its possible implication in neurodevelopmental -

Measurement of Sylvian Fissure Asymmetry and Occipital Bending in Humans and Pan Troglodytes
Edinburgh Research Explorer Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes Citation for published version: Hou, L, Xiang, L, Crow, T, Leroy, F, Rivière, D, Mangin, J-F & Roberts, N 2018, 'Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes', NeuroImage. https://doi.org/10.1016/j.neuroimage.2018.08.045 Digital Object Identifier (DOI): 10.1016/j.neuroimage.2018.08.045 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: NeuroImage General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 Accepted Manuscript Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes Lewis Hou, Li Xiang, Timothy Crow, François Leroy, Denis Rivière, Jean- François Mangin, Neil Roberts PII: S1053-8119(18)30743-2 DOI: 10.1016/j.neuroimage.2018.08.045 Reference: YNIMG 15206 To appear in: NeuroImage Received Date: 10 September 2017 Revised Date: 15 August 2018 Accepted Date: 17 August 2018 Please cite this article as: Hou, L., Xiang, L., Crow, T., Leroy, Franç., Rivière, D., Mangin, Jean-.Franç., Roberts, N., Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes, NeuroImage (2018), doi: 10.1016/j.neuroimage.2018.08.045. -

Adolescent Brain Development
ADOLESCENT BRAIN DEVELOPMENT Adolescence is a period of physical, mental, and Youth, even in their late teens, do not have emotional development, generally occurring the same ability as adults to make mature between the ages of 12 and 18, often accompanied decisions. by distinct behavioral changes.¹ Engaging in reckless actions during adolescence is Neuroscience research shows that there are socially normative behavior.⁹ However, although physical differences between the brains of crimes peak around late adolescence, they begin a adults and youth. steep decline into adulthood.¹⁰ It is harder for adolescents to exercise self-control According to recent findings, the human brain does than it is for adults.¹¹ In fact, it is unreasonable to not reach full maturity until at least the mid-20s.² expect that people younger than 18 will have a fully The specific changes that follow young adulthood are formed ability to resist impulses.¹² not yet well studied, but it is known that they involve Adolescents and adults think differently in terms of increased myelination and continued adding and risks and rewards when considering alternative pruning of neurons.³ choices. In particular, because of shifts in dopamine The prefrontal cortex of the brain is crucial for production, kids are more likely to place greater weighing risk vs. reward, future planning, impulse weight on rewards than on risks when making such a control, and is critical for a person to be able to make choice.¹³ This type of decision-making is likely to lead rational decisions.⁴ However, it is one of the last parts to risky behaviors.¹⁴ of the brain to develop and is still not fully mature by Adolescents are less likely to consider the long-term late adolescence.⁵ consequences of the actions they choose because The limbic system, which assists in processing and their capacity for thinking and planning for the future managing emotions, is still maturing during is still developing.¹⁵ adolescence. -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface.