COVID-19 Vaccine Astrazeneca Real-World Evidence Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematic Booster After Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure
Article Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure Perrine Parize 1,*, Jérémie Sommé 2 , Laura Schaeffer 3, Florence Ribadeau-Dumas 1, Sheherazade Benabdelkader 1, Agnès Durand 4, Arnaud Tarantola 1, Johann Cailhol 5, Julia Goesch 5, Lauriane Kergoat 1 , Anne-Sophie Le Guern 6, Marie-Laurence Mousel 2, Laurent Dacheux 1 , Paul-Henri Consigny 5, Arnaud Fontanet 3,7, Beata Francuz 2 and Hervé Bourhy 1 1 Institut Pasteur, Unit Lyssavirus Epidemiology and Neuropathology, National Reference Center for Rabies and WHO Collaborating Centre for Reference and Research on Rabies, 75015 Paris, France; fl[email protected] (F.R.-D.); [email protected] (S.B.); [email protected] (A.T.); [email protected] (L.K.); [email protected] (L.D.); [email protected] (H.B.) 2 Institut Pasteur, Occupational Health Department, 75015 Paris, France; [email protected] (J.S.); [email protected] (M.-L.M.); [email protected] (B.F.) 3 Institut Pasteur, Emerging Diseases Epidemiology Unit, Centre for Global Health Research and Education, 75015 Paris, France; [email protected] (L.S.); [email protected] (A.F.) 4 Laboratoire Cerballiance, 75017 Paris, France; [email protected] 5 Citation: Parize, P.; Sommé, J.; Institut Pasteur, Centre Médical, Centre d’Infectiologie Necker-Pasteur, 75015 Paris, France; Schaeffer, L.; Ribadeau-Dumas, F.; [email protected] (J.C.); [email protected] (J.G.); [email protected] (P.-H.C.) 6 Benabdelkader, S.; Durand, A.; Institut Pasteur, Laboratory of the Medical Center, 75015 Paris, France; [email protected] 7 Conservatoire National des Arts et Métiers, 75003 Paris, France Tarantola, A.; Cailhol, J.; Goesch, J.; * Correspondence: [email protected]; Tel.: +33-140-613-436 Kergoat, L.; et al. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Third Quarter 2020
March 31, 2020 Third Quarter 2020 Corporate update and financial results November 10, 2020 Forward-looking statements Various statements in this slide presentation concerning the future expectations of BioNTech, its plans and prospects, including the Company's views with respect to the potential for mRNA and other pipeline therapeutics; BioNTech's efforts to combat COVID-19; the collaborations between BioNTech and Pfizer and Fosun to develop a potential COVID-19 vaccine; our expectations regarding the potential characteristics of BNT162b2 in our continuing Phase 2/3 trial and/or in commercial use based on data observations to date; the expected timepoint for additional readouts on efficacy data of BNT162b2 in our Phase 2/3 trial; the nature of the clinical data for BNT162, BNT311 and our other product candidates, which is subject to ongoing peer review, regulatory review and market interpretation; the timing for submission of data for, or receipt of, any potential approval or Emergency Use Authorization with respect to our BNT162 program; the timing for submission of BNT162 manufacturing data to the FDA; the ability of BioNTech to supply the quantities of BNT162 to support clinical development and, if approved, market demand, including our production estimates for 2020 and 2021 and orders received to-date; the planned next steps in BioNTech's pipeline programs and specifically including, but not limited to, statements regarding plans to initiate clinical trials of BioNTech's product candidates and expectations for data announcements with -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -
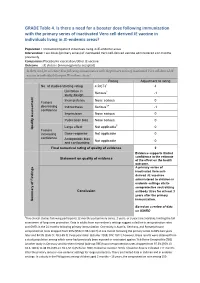
GRADE Table 4. Is There a Need for a Booster Dose Following Immunization Wi
GRADE Table 4. Is there a need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Population : Immunocompetent individuals living in JE-endemic areas Intervention: Two doses (primary series) of inactivated Vero cell-derived vaccine administered ≥12 months previously Comparison: Placebo/no vaccination/other JE vaccine Outcome : JE disease (immunogenicity accepted) Is there need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Rating Adjustment to rating No. of studies/starting rating 4 RCTs1 4 Limitation in 2 Serious -1 study design Inconsistency None serious 0 Factors decreasing Indirectness Serious3,4 -1 confidence Imprecision None serious 0 Publication bias None serious 0 Large effect Not applicable5 0 Quality Assessment Quality Factors increasing Dose-response Not applicable 0 confidence Antagonistic bias Not applicable 0 and confounding Final numerical rating of quality of evidence 2 Evidence supports limited confidence in the estimate Statement on quality of evidence of the effect on the health outcome. A primary series of inactivated Vero cell- derived JE vaccines administered to children in endemic settings elicits seroprotective neutralizing Conclusion antibody titres for at least 3 years after the primary immunization. Summary of Findings of Summary Based on a review of data on IXIARO 1Five clinical studies following participants 12 months post-primary series, 2 years, or 3 years are available, limiting the full assessment of long-term protection. Data in adults from non-endemic settings suggest a decline in seroprotection rates and GMTs in the 24 months following primary immunization. -

Pfizer-Biontech COVID-19 Vaccine Consent and Screening Form For
Page 1 of 2 Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 VACCINE, mRNA) Consent and Screening Form for Individuals Under 18 Years of Age SECTION 1: INFORMATION ABOUT MINOR CHILD TO RECEIVE VACCINE (PLEASE PRINT) MINOR’S NAME (Last) (First) (M.I.) MINOR’S DATE OF BIRTH (MM/DD/YEAR): MINOR’S RACE ETHNICITY Is Minor a person White Black Asian Native American or Alaska Native Hispanic with a disability? Native Hawaiian or Pacific Islander Non-Hispanic YES NO PARENT/LEGAL GUARDIAN’S NAME (First) (M.I.) MINOR’S AGE: MINOR’S (Last) GENDER: M / F ADDRESS PARENT/GUARDIAN DAYTIME PHONE NUMBER AND MOBILE NUMBER: CITY STATE ZIP PARENT/GUARDIAN EMAIL ADDRESS: SECTION 2: SCREENING FOR VACCINE ELIGIBILITY The following questions will help determine if there is any reason your child should not get the COVID-19 vaccine. If you answer “yes” to any question, it does not necessarily mean that your child should not be vaccinated. It just means additional questions may be asked. If a question is not clear, please ask your healthcare provider to explain it. YES NO UNKNOWN 1. Is your child currently feeling sick or ill? 2. Has your child ever received a dose of the COVID-19 vaccine? If yes, which vaccine? Moderna; Pfizer; Janssen (Johnson & Johnson); Comirnaty; another brand of vaccine: _________________ Date: ___________ 3. Has your child ever had an allergic reaction to: (This would include a severe allergic reaction [e.g., anaphylaxis] that required treatment with epinephrine or EpiPen® or that caused you to go to the hospital. It would also include an allergic reaction that caused hives, swelling, or respiratory distress, including wheezing.) A component of a COVID-19 vaccine, including any of the following: o Polyethylene glycol (PEG), which is found in some medications, such as laxatives and preparations for colonoscopy procedures? o Polysorbate, which is found in some vaccines, film coated tablets, and intravenous steroids? A previous dose of COVID-19 vaccine? 4. -

Hepatitis B and Healthcare Personnelq &A IAC Answers Frequently Asked Questions About How to Protect Healthcare Personnel
Hepatitis B and Healthcare PersonnelQ &A IAC answers frequently asked questions about how to protect healthcare personnel Experts from the Immunization Action Engerix-B (GSK) or Recombivax HB (Merck) should be vaccinated against hepatitis B if Coalition (IAC) answer your questions may be completed with Heplisav-B. However, they haven’t been previously vaccinated. about hepatitis B (HepB) vaccine. You’ll data are limited on the safety and immunoge- Receipt of the vaccine is not a reason to dis- nicity effects when Heplisav-B is interchanged continue breast-feeding. find additional &Q As about hepatitis B with hepatitis B vaccines from other manufac- There are no clinical studies of Heplisav-B in vaccine on the “Ask the Experts” section turers. When feasible, the same manufactur- pregnant women. Available human data on of immunize.org at www.immunize.org/ er’s vaccines should be used to complete the Heplisav-B administered to pregnant women askexperts/experts_hepb.asp series. However, vaccination should not be are insufficient to assess vaccine-associated deferred when the manufacturer of the previ- risks in pregnancy. Until safety data are avail- ously administered vaccine is unknown or able for Heplisav-B, providers should con- Hepatitis B Vaccination when the vaccine from the same manufac- tinue to vaccinate pregnant women needing turer is unavailable. hepatitis B vaccination with a vaccine from Which people who work in healthcare settings The 2-dose hepatitis B vaccine series only a different manufacturer. need hepatitis B vaccine? applies when both doses in the series consist Is there a recommendation for routine booster The Occupational Safety and Health Adminis- of Heplisav-B. -

Developing a Vaccine Against HIV Infection
Developing a vaccine against HIV infection KEY POINTS Researchers have been working on an HIV vaccine since the 1980s, but progress towards an effective vaccine has been much slower than anticipated. Finding at least a partially effective vaccine remains of critical importance for the HIV response. The biggest reduction in new infections would be achieved by a combination of PrEP, universal antiretroviral treatment for people already living with HIV, and a vaccine.1 An HIV vaccine is a more realistic prospect today than a decade ago and an optimistic forecast of HIV vaccine availability is that one might be available by 2030. Explore this page to find out more about the need for a vaccine against HIV, challenges in vaccine development, progress in developing a vaccine and achieving an effective vaccine for HIV. What is an HIV vaccine? Today, an effective vaccine against HIV does not exist. A vaccine that can prevent infection would teach the immune system to respond to HIV by making antibodies that can bind to the virus and stop it from infecting cells, or by promoting other immune responses that kill the virus. No vaccine is 100% effective, and this is likely to be the same for HIV. Some people who receive a vaccine will not respond strongly enough to the vaccine and will not be protected, as in the case of the seasonal flu vaccine. But finding at least a partially effective vaccine remains of critical importance for the HIV response, as all successful disease elimination strategies have included a vaccine among their arsenal. The need for a vaccine against HIV UNAIDS estimates that 1.8 million people became infected with HIV in 2017, 36.9 million people were living with HIV and 21.7 million were receiving antiretroviral therapy. -

Vaccinations for Pregnant Women the Table Below Shows Which Vaccinations You May Or May Not Need During Your Pregnancy
Vaccinations for Pregnant Women The table below shows which vaccinations you may or may not need during your pregnancy. Vaccine Do you need it during your pregnancy? Influenza Yes! You need a flu shot every fall (or even as late as winter or spring) for your protection and for the protection of your baby and others around you. It’s safe to get the vaccine at any time during your pregnancy. Tetanus, diphtheria, Yes! Women who are pregnant need a dose of Tdap vaccine (the adult whooping cough vaccine) during each pregnancy, prefer- whooping cough ably in the early part of the third trimester. It’s safe to be given during pregnancy and will help protect your baby from whooping (pertussis) cough in the first few months after birth when he or she is most vulnerable. After Tdap, you need a Tdap or Td booster dose every Tdap, Td 10 years. Consult your healthcare provider if you haven’t had at least 3 tetanus- and diphtheria-toxoid containing shots some- time in your life or if you have a deep or dirty wound. Human No. This vaccine is not recommended to be given during pregnancy, but if you inadvertently receive it, this is not a cause for papillomavirus concern. HPV vaccine is recommended for all people age 26 or younger, so if you are in this age group, make sure you are HPV vaccinated before or after your pregnancy. People age 27 through 45 may also be vaccinated against HPV after discussion with their healthcare provider. The vaccine is given in 2 or 3 doses (depending on the age at which the first dose is given) over a 6-month period.