How to Set up a Reflux Apparatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Single Pass Cooling Systems
A Sustainable Alternative to Single Pass Cooling Systems Amorette Getty, PhD Co-Director, LabRATS What is Single Pass Cooling? ● Used for distillation/reflux condensers, ice maker condensers, autoclaves, cage washers… ● Use a continuous flow of water from faucet to sewer o 0.25-2 gallons per minute, o up to 1,000,000 gallons of water/year if left on continuously Single Pass Cooling: Support ● “U.S. Environmental Protection Agency has ranked the elimination of single-pass cooling systems #4 on its list of top ten water management techniques” -Steve Buratto, Chair of Chemistry and Biochemistry department NOT Just a Sustainability Issue! California Nanosystems Institute (CNSI) flood in a second-floor laboratory in July, 2014. Water ran at ~1-2 gallons per minute for 6-10 hours, overflowing into a ground floor electron microscopy lab CNSI Flood Losses ● $ 2.2 Million in custom built research equipment ● Research shutdown for 4 months ● over 200 hours of staff time ● Insurance no longer covering this type of incident again Campus Responses to the Incident • CNSI ban on single pass cooling systems • TGIF grant financial incentive for optional change of single replacement systems • Chemistry and Material Research Laboratory (MRL) • Additional Grants being sought • Conversation at Campus and UC-level to regulate and replace these types of systems. Seeking Allies… ● “The replacement of single-pass cooling systems with closed-loop and water free systems would save water and represent a major advance in the sustainability efforts of the campus.” -Steve Buratto, Chair of Chemistry and Biochemistry department Barriers to Replacement ● “...major obstacle to replacing the single-pass cooling systems with closed-loop and water- free systems is cost. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -

22 Bull. Hist. Chem. 8 (1990)
22 Bull. Hist. Chem. 8 (1990) 15 nntn Cn r fr l 4 0 tllOt f th r h npntd nrpt ltd n th brr f th trl St f nnlvn n thr prt nd ntn ntl 1 At 1791 1 frn 7 p 17 AS nntn t h 3 At 179 brr Cpn f hldlph 8Gztt f th Untd Stt Wdnd 3 l 1793 h ntr nnnnt rprdd n W Ml "njn h Cht"Ch 1953 37-77 h pn pr dtd 1 l 179 nd nd b Gr Whntn th frt ptnt d n th Untd Stt S M ntr h rt US tnt" Ar rt Invnt hn 199 6(2 1- 19 ttrfld ttr fnjn h Arn hl- phl St l rntn 1951 pp 7 9 20h drl Gztt 1793 (20 Sptbr Qtd n Ml rfrn 1 1 W Ml rfrn 1 pp 7-75 William D. Williams is Professor of Chemistry at Harding r brt tr University, Searcy, Al? 72143. He collects and studies early American chemistry texts. Wyndham D. Miles. 24 Walker their British cultural heritage. To do this, they turned to the Avenue, Gaithersburg, MD 20877, is winner of the 1971 schools (3). This might explain why the Virginia assembly Dexter Award and is currently in the process of completing the took time in May, 1780 - during a period when their highest second volume of his biographical dictionary. "American priority was the threat of British invasion following the fall of Chemists and Chemical Engineers". Charleston - to charter the establishment of Transylvania Seminary, which would serve as a spearhead of learning in the wilderness (1). -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -
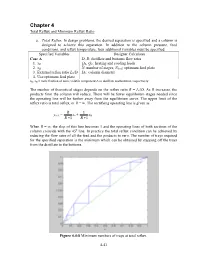
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

HORIZONTAL BOTTLE COOLER EBC Series Model: EBC50
Project Name: HORIZONTAL BOTTLE COOLER Project Location: Model #: Quantity: Available Warehouse: EBC Series Model: EBC50 Refrigeration System ∙ Side mounted, self-contained and fully detachable Blizzard R290 condensing unit uses environmentally friendly, EPA-compliant R290 refrigerant with zero (0) Ozone Depletion Potential (ODP) and three (3) Global Warming Potential (GWP). Blizzard R290 is easily replaceable and requires no on-site brazing. ∙ Electronically commutated (ECM) fan motors achieve rapid cooling with less energy consumption. ∙ Full-length air duct system ensures optimal circulation of cold air. ∙ Time-initiated and temperature-terminated auto defrost cycle for seamless operation. ∙ Large capacity, corrosion-resistant condenser and evaporator coils. ∙ Self-maintaining, energy-efficient condensate drain pan requires no external drains or electric heaters. ∙ High performance, auto-reverse condenser fan motor supports compressor ventilation and condenser coil cleaning. Refer to owner’s manual for full maintenance instructions. ∙ Pre-wired and ready to plug, 115V/60Hz/1Ph, NEMA 5-15P. Cabinet Construction ∙ Heavy duty stainless steel countertop and rails with textured laminate, black vinyl exterior. ∙ Open spaced interior with no walls between compartments. ∙ Galvanized steel interior. ∙ 1 3/4" thick high density polyurethane insulation. ∙ Built-in caster thread receptacles for all models. Lighting ∙ Shielded LED bar lighting with on/off switch provides bright, high color illumination at lower heat output. Bottle Cap Opener ∙ Front mounted bottle cap opener and cap catcher are removable for ease of cleaning. Lids ∙ Heavy duty stainless steel exterior and galvanized steel interior. ∙ 3/4" thick high density polyurethane insulation. ∙ Removable ratchet locks keep your items safe from theft. Shelving ∙ Horizontal epoxy coated, steel wire shelves for bottom air circulation (see table for quantity). -

LSC- DTS Laboratory Sample Condenser
Quality System Certified ISO 9001 LSC-DTS Sample Condenser Rev.2.doc Page 1 of 1 LSC- DTS Laboratory Sample Condenser LSC- DTS Laboratory Sample Condenser This LSC unit range of stainless steel tube shaft sterile heat exchangers with Double Tube Sheet (DTS) have been designed to allow Clean Steam (CS) and Water For Injection (WFI) samples to be taken quickly and easly whilst mantaining a sterile testing environment. LSC are ideal to be mounted at the sampling point and can be operated with either mains or chilled water as the cooling medium. Availability of aseptic sample valve allow fine control of sample flow during testing The LSC can be sterilised in situ-on-line, thus ensuring continuity of samples regardless of testing frequency, ideal for fluids in pharmaceutical and purity systems applications. Typical applications: Steam sampling In-line conductivity monitoring Point of use cooling Feature offered by the unit include: AISI 316L (1.4404) stainless steel construction Double Tube Sheet (DTS) Meets FDA and 3A specifications Full material traceability Self draining design Compact, easy to install Fully sterilisable/autoclavable Sample Condenser operation The medium to be condensed/cooled passes throught the tube side. Typically a regulating valve will be used to throttle the sample medium flow. Cooling water is channelled countercurrent inside the shell in order to ensure maximum efficiency. The heat energy of the sample medium is absorbed by the flowing cooling water, resulting in a drop in the sample temperature. Where steam is the sample medium, the cooling water will firstly absorb the steam’s latent heat content, condensing it back to water. -

Members/Downloads/Docs/Distilling Instructions Super Reflux Condenser.Pdf
Distilling Instructions (Super Reflux Condenser) 9 Adams Road Palmerston NT 0830 Tel: (08) 8932 7700 Website: www.ozbrew.com Shop 17 Homemaker Village Millner NT Fax: (08) 8932 7622 Email: [email protected] Before reading these instructions, make sure you have read the information sheet called “Distilling Overview” which is also available in the downloads section of the Oz Brew website. The “Distilling Overview” sheet will provide useful information about the legality of stills and their usages, as well as outlining some good overall basics that will help provide essential information about this process. There are many types of stills, boilers, condensers & carbon filters available on the market. Different products and brands, may use different instructions. These instructions cover one of the most practical methods of distilling using a 25L boiler, a (super) reflux condenser and a Still Spirits Z- carbon filter, which we believe is one of the most effective value for money packages available. Read through all of your instructions including the Helpful Hints in each section before proceeding through the steps in each process. This will ensure you are aware of any tips that may make the process 25 Litre Boiling Pot Z Carbon Filter Super Reflux Condenser easier for you. (3 in 1 Fermenter) Typically produces a high yield of approximately 4-5L @ 80% - 90% V. Stage 1 - Sterilisation 1. Everything used in the fermentation process must be cleaned and sterilised before and after use. This includes the 3 in 1 fermenter, airlock and stirrer. 2. For optimum cleaning, we recommend Combo Cleaner Washing / Steriliser. Wash through all of your equipment with this solution and rinse thoroughly with cold tap water. -

Grignard Synthesis of Triphenylmethanol Reactions That Form Carbon-Carbon Bonds Are Among the Most Useful to the Synthetic Organic Chemist
1 Experiment 12: Grignard Synthesis of Triphenylmethanol Reactions that form carbon-carbon bonds are among the most useful to the synthetic organic chemist. In 1912, Victor Grignard received the Nobel prize in chemistry for his discovery of a new series of reactions that result in the formation of a carbon-carbon bond. A Grignard synthesis first involves the preparation of an organomagnesium reagent via the reaction of an alkyl bromide with magnesium metal: δ– δ+ R Br + Mg R MgBr The resulting “Grignard reagent” acts as both a good nucleophile and a strong base. Its nucleophilic character allows it to react with the electrophilic carbon in a carbonyl group, thus forming the carbon-carbon bond. Its basic property means that it will react with acidic compounds, such as carboxylic acids, phenols, thiols and even alcohols and water; therefore, reaction conditions must be free from acids and strictly anhydrous. Grignard reagents will also react with oxygen to form hydroperoxides, thus they are highly unstable when exposed to the atmosphere and are generally not isolated from solution. For a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a Grignard synthesis. Vapors from the highly volatile solvent help to prevent oxygen from reaching the reaction solution. In addition, evidence suggests that the ether molecules actually coordinate with and help stabilize the Grignard reagent: Et Et O R Mg Br O Et Et The magnesium metal used in the synthesis contains a layer of oxide on the surface that prevents it from reacting with the alkyl bromide. The pieces of metal must be gently scratched while in the ether solution to expose fresh surface area so that the reaction can commence.