Genetic Bases of Craniosynostoses: an Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prenatal Ultrasonography of Craniofacial Abnormalities
Prenatal ultrasonography of craniofacial abnormalities Annisa Shui Lam Mak, Kwok Yin Leung Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China REVIEW ARTICLE https://doi.org/10.14366/usg.18031 pISSN: 2288-5919 • eISSN: 2288-5943 Ultrasonography 2019;38:13-24 Craniofacial abnormalities are common. It is important to examine the fetal face and skull during prenatal ultrasound examinations because abnormalities of these structures may indicate the presence of other, more subtle anomalies, syndromes, chromosomal abnormalities, or even rarer conditions, such as infections or metabolic disorders. The prenatal diagnosis of craniofacial abnormalities remains difficult, especially in the first trimester. A systematic approach to the fetal Received: May 29, 2018 skull and face can increase the detection rate. When an abnormality is found, it is important Revised: June 30, 2018 to perform a detailed scan to determine its severity and search for additional abnormalities. Accepted: July 3, 2018 Correspondence to: The use of 3-/4-dimensional ultrasound may be useful in the assessment of cleft palate and Kwok Yin Leung, MBBS, MD, FRCOG, craniosynostosis. Fetal magnetic resonance imaging can facilitate the evaluation of the palate, Cert HKCOG (MFM), Department of micrognathia, cranial sutures, brain, and other fetal structures. Invasive prenatal diagnostic Obstetrics and Gynaecology, Queen Elizabeth Hospital, Gascoigne Road, techniques are indicated to exclude chromosomal abnormalities. Molecular analysis for some Kowloon, Hong Kong SAR, China syndromes is feasible if the family history is suggestive. Tel. +852-3506 6398 Fax. +852-2384 5834 E-mail: [email protected] Keywords: Craniofacial; Prenatal; Ultrasound; Three-dimensional ultrasonography; Fetal structural abnormalities This is an Open Access article distributed under the Introduction terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/ licenses/by-nc/3.0/) which permits unrestricted non- Craniofacial abnormalities are common. -

MR Imaging of Fetal Head and Neck Anomalies
Neuroimag Clin N Am 14 (2004) 273–291 MR imaging of fetal head and neck anomalies Caroline D. Robson, MB, ChBa,b,*, Carol E. Barnewolt, MDa,c aDepartment of Radiology, Children’s Hospital Boston, 300 Longwood Avenue, Harvard Medical School, Boston, MA 02115, USA bMagnetic Resonance Imaging, Advanced Fetal Care Center, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA cFetal Imaging, Advanced Fetal Care Center, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA Fetal dysmorphism can occur as a result of var- primarily used for fetal MR imaging. When the fetal ious processes that include malformation (anoma- face is imaged, the sagittal view permits assessment lous formation of tissue), deformation (unusual of the frontal and nasal bones, hard palate, tongue, forces on normal tissue), disruption (breakdown of and mandible. Abnormalities include abnormal promi- normal tissue), and dysplasia (abnormal organiza- nence of the frontal bone (frontal bossing) and lack of tion of tissue). the usual frontal prominence. Abnormal nasal mor- An approach to fetal diagnosis and counseling of phology includes variations in the size and shape of the parents incorporates a detailed assessment of fam- the nose. Macroglossia and micrognathia are also best ily history, maternal health, and serum screening, re- diagnosed on sagittal images. sults of amniotic fluid analysis for karyotype and Coronal images are useful for evaluating the in- other parameters, and thorough imaging of the fetus tegrity of the fetal lips and palate and provide as- with sonography and sometimes fetal MR imaging. sessment of the eyes, nose, and ears. -

Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

A Heads up on Craniosynostosis
Volume 1, Issue 1 A HEADS UP ON CRANIOSYNOSTOSIS Andrew Reisner, M.D., William R. Boydston, M.D., Ph.D., Barun Brahma, M.D., Joshua Chern, M.D., Ph.D., David Wrubel, M.D. Craniosynostosis, an early closure of the growth plates of the skull, results in a skull deformity and may result in neurologic compromise. Craniosynostosis is surprisingly common, occurring in one in 2,100 children. It may occur as an isolated abnormality, as a part of a syndrome or secondary to a systemic disorder. Typically, premature closure of a suture results in a characteristic cranial deformity easily recognized by a trained observer. it is usually the misshapen head that brings the child to receive medical attention and mandates treatment. This issue of Neuro Update will present the diagnostic features of the common types of craniosynostosis to facilitate recognition by the primary care provider. The distinguishing features of other conditions commonly confused with craniosynostosis, such as plagiocephaly, benign subdural hygromas of infancy and microcephaly, will be discussed. Treatment options for craniosynostosis will be reserved for a later issue of Neuro Update. Types of Craniosynostosis Sagittal synostosis The sagittal suture runs from the anterior to the posterior fontanella (Figure 1). Like the other sutures, it allows the cranial bones to overlap during birth, facilitating delivery. Subsequently, this suture is the separation between the parietal bones, allowing lateral growth of the midportion of the skull. Early closure of the sagittal suture restricts lateral growth and creates a head that is narrow from ear to ear. However, a single suture synostosis causes deformity of the entire calvarium. -

Craniofacial Syndromes: Crouzon, Apert, Pfeiffer, Saethre-Chotzen, and Carpenter Syndromes, Pierre Robin Syndrome, Hemifacial Deformity 10/4/17, 4�06 PM
Craniofacial Syndromes: Crouzon, Apert, Pfeiffer, Saethre-Chotzen, and Carpenter Syndromes, Pierre Robin Syndrome, Hemifacial Deformity 10/4/17, 406 PM Craniofacial Syndromes Updated: Feb 21, 2016 Author: Kongkrit Chaiyasate, MD, FACS; Chief Editor: Jorge I de la Torre, MD, FACS more... Crouzon, Apert, Pfeiffer, Saethre-Chotzen, and Carpenter Syndromes Crouzon Syndrome Crouzon syndrome was first described in 1912. Inheritance Inheritance is autosomal dominant with virtually complete penetrance. It is caused by multiple mutations of the fibroblast growth factor receptor 2 gene, FGFR2. [1, 2, 3] Features Features of the skull are variable. The skull may have associated brachycephaly, trigonocephaly, or oxycephaly. These occur with premature fusion of sagittal, metopic, or coronal sutures, with the coronal sutures being the most common. In addition, combinations of these deformities may be seen. [4] See the image below. http://emedicine.medscape.com/article/1280034-overview PaGe 1 of 61 Craniofacial Syndromes: Crouzon, Apert, Pfeiffer, Saethre-Chotzen, and Carpenter Syndromes, Pierre Robin Syndrome, Hemifacial Deformity 10/4/17, 406 PM Typical appearance of a patient with Crouzon syndrome, with maxillary retrusion, exorbitism, and pseudoprognathism. Anteroposterior view. View Media Gallery The orbits are shallow with resulting exorbitism, which is due to anterior positioning of the greater wing of the sphenoid. The middle cranial fossa is displaced anteriorly and inferiorly, which further shortens the orbit anteroposteriorly. The maxilla is foreshortened, causing reduction of the orbit anteroposteriorly. All these changes result in considerable reduction of orbital volume and resultant significant exorbitism. In severe cases, the lids may not close completely. The maxilla is hypoplastic in all dimensions and is retruded. -

Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017
Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017 Participating members of the Birth Defects Definitions Group: Lorenzo Botto (UT) John Carey (UT) Cynthia Cassell (CDC) Tiffany Colarusso (CDC) Janet Cragan (CDC) Marcia Feldkamp (UT) Jamie Frias (CDC) Angela Lin (MA) Cara Mai (CDC) Richard Olney (CDC) Carol Stanton (CO) Csaba Siffel (GA) Table of Contents LIST OF BIRTH DEFECTS ................................................................................................................................................. I DETAILED DESCRIPTIONS OF BIRTH DEFECTS ...................................................................................................... 1 FORMAT FOR BIRTH DEFECT DESCRIPTIONS ................................................................................................................................. 1 CENTRAL NERVOUS SYSTEM ....................................................................................................................................... 2 ANENCEPHALY ........................................................................................................................................................................ 2 ENCEPHALOCELE ..................................................................................................................................................................... 3 HOLOPROSENCEPHALY............................................................................................................................................................. -

A Prospective Study of Cranial Deformity and Delayed Development in Children
sustainability Article A Prospective Study of Cranial Deformity and Delayed Development in Children Josefa González-Santos 1, Jerónimo J. González-Bernal 1,* , Raquel De-la-Fuente-Anuncibay 1 , José M. Aguilar-Parra 2,*, Rubén Trigueros 2,*, Raúl Soto-Cámara 1 and Remedios López-Liria 3 1 Department of Psychology, University of Burgos, 09001 Burgos, Spain; [email protected] (J.G.-S.); [email protected] (R.D.-l.-F.-A.); [email protected] (R.S.-C.) 2 Department of Psychology, Health Research Centre, University of Almeria, 04120 Almeria, Spain 3 Department of Nursing, Physiotherapy and Medicine, Health Research Centre, University of Almería, 04120 Almeria, Spain; [email protected] * Correspondence: [email protected] (J.J.G.-B.); [email protected] (J.M.A.-P.); [email protected] (R.T.) Received: 27 January 2020; Accepted: 2 March 2020; Published: 4 March 2020 Abstract: Plagiocephaly, the most common form of cranial deformity, has become more prevalent in recent years. Many authors have described a number of sequelae of poorly defined etiologies, although several gaps exist in their real scope. This study aimed to analyze the effects of physiotherapy treatments and cranial orthoses on the psychomotor development of infants with cranial deformities, complemented by protocolized postural exercises applied by the family. This prospective study on different developmental areas included a sample of 48 breastfeeding infants aged 6 to 18 months who presented with plagiocephaly (flat head syndrome). The Brunet–Lézine scale was used to perform three tests for assessing the psychomotor development of infants, thus offering a measure for global development. The results suggest that plagiocephaly is a marker for the risk of delayed development, particularly in motor and language areas. -

Craniosynostosis
European Journal of Human Genetics (2011) 19, 369–376 & 2011 Macmillan Publishers Limited All rights reserved 1018-4813/11 www.nature.com/ejhg PRACTICAL GENETICS In association with Craniosynostosis Craniosynostosis, defined as the premature fusion of the cranial sutures, presents many challenges in classification and treatment. At least 20% of cases are caused by specific single gene mutations or chromosome abnormalities. This article maps out approaches to clinical assessment of a child presenting with an unusual head shape, and illustrates how genetic analysis can contribute to diagnosis and management. In brief Apart from the genetic implications, it is important to recog- nise cases with a genetic cause because they are more likely to Craniosynostosis is best managed in a multispecialty tertiary be associated with multiple suture synostosis and extracranial referral unit. complications. Single suture synostosis affects the sagittal suture most com- Genes most commonly mutated in craniosynostosis are monly, followed by the coronal, metopic and lambdoid sutures. FGFR2, FGFR3, TWIST1 and EFNB1. Both environmental factors (especially intrauterine fetal head As well as being associated with syndromes, some clinically constraint) and genes (single gene mutations, chromosome non-syndromic synostosis (usually affecting the coronal abnormalities and polygenic background) predispose to cra- suture) can be caused by single gene mutations, particularly niosynostosis. the Pro250Arg mutation in FGFR3. Most genetically determined craniosynostosis is characterised In severe cases, initial care should be directed towards main- by autosomal dominant inheritance, but around half of cases tenance of the airway, support of feeding, eye protection and are accounted for by new mutations. treatment of raised intracranial pressure. -

Isolated Fetal Neural Tube Defects Associate with Increased Risk of Placental Pathology: Evidence from the Collaborative
medRxiv preprint doi: https://doi.org/10.1101/2021.03.16.21253704; this version posted March 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Isolated fetal neural tube defects associate with increased risk of placental pathology: evidence from the Collaborative Perinatal Project. Marina White1, David Grynspan2,3, Tim Van Mieghem4, *Kristin L Connor1 1Health Sciences, Carleton University, Ottawa, ON K1S 5B6, Canada; 2Vernon Jubilee Hospital, Vernon, BC V1T 5L2, Canada; 3Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC V6T 1Z7, Canada; 4Department of Obstetrics and Gynaecology, Mount Sinai Hospital, Toronto, ON M5G 1X5, Canada *Corresponding author: Dr. Kristin Connor Department of Health Sciences, Carleton University, Ottawa, Ontario, Canada, K1S5B6 E: [email protected], Tel.: +1 613-520-2600 ext. 4202 Abstract Objective: To compare placental pathology and fetal growth in pregnancies with an isolated fetal neural tube defect (NTD; cases) to those without congenital anomalies (controls). We hypothesised that cases would be at an increased risk of placental pathology and poorer anthropometric outcomes at birth compared to controls Methods: We performed a matched case-cohort study using data from the Collaborative Perinatal Project. Cases (n=74) and controls (n=148) were matched (1:2 ratio) for maternal pre-pregnancy BMI, maternal race, infant sex, gestational age at birth and study site. Primary outcomes were placental characteristics (weight and size measurements, pathology). Secondary outcomes were infant birth outcomes. -

Kraniofaciale Malformasjoner V02
2/1/2021 Kraniofaciale malformasjoner v02 Avdeling for medisinsk genetikk Kraniofaciale malformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). For noen gener ligger alle ekson i områder med segmentale duplikasjoner: UBB Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Gen Gen Ekson (HGNC (HGNC Transkript affisert av Ekson** Fenotype symbol) ID) segdup* ALPL 438 NM_000478.4 2-12 Hypophosphatasia, childhood OMIM ALX3 449 NM_006492.2 1-4 Frontonasal dysplasia 1 OMIM ALX4 450 NM_021926.3 1-4 {Craniosynostosis 5, susceptibility to} OMIM Parietal foramina 2 OMIM Frontonasal dysplasia 2 OMIM ASXL1 18318 NM_015338.5 1-12 Bohring-Opitz syndrome OMIM ATR 882 NM_001184.3 1-47 Seckel syndrome 1 OMIM BMP4 1071 NM_001202.3 3-4 Orofacial cleft 11 OMIM file:///data/KFM_v02-web.html 1/10 2/1/2021 Kraniofaciale malformasjoner v02 Gen Gen Ekson (HGNC (HGNC Transkript affisert av Ekson** Fenotype symbol) -

Diagnosing and Treating Deformational Plagiocephaly
Nonprofit Organization U.S. Postage PAID Twin Cities, MN Study of Nearly 10,000 Patients Who Have VOLUME 22, NUMBER 5 2013 200 University Ave. E. Permit No. 5388 St. Paul, MN 55101 Head Shape Conditions Yields Valuable 651-291-2848 www.gillettechildrens.org Insights ADDRESS SERVICE VOLUME 22, NUMBER 5 2013 REQUESTED Gillette’s craniofacial program launched nearly 15 years ago under the guidance of Robert Wood, M.D. Our program serves patients from the upper Midwest A Pediatric Perspective focuses on specialized topics in pediatrics, orthopedics, and across the nation who have complex congenital neurology, neurosurgery and rehabilitation medicine. conditions ranging from cleft lip and palate to cranio- Diagnosing and Treating KEY INSIGHTS synostosis. We also care for patients who have rare To subscribe to or unsubscribe from syndromes that affect facial features and head shapes, A Pediatric Perspective, please send an Deformational Plagiocephaly, email to [email protected]. including Apert, Pfieffer and Crouzon syndromes. ■ Craniosynostosis, a condition in which an infant’s cranial bones fuse Editor-in-Chief – Steven Koop, M.D. Torticollis and Craniosynostosis prematurely, should be repaired when Because we have one of the largest programs in the Editor – Ellen Shriner Designers – Becky Wright, Kim Goodness the child is 3 to 6 months old. country—treating nearly 10,000 patients in 15 years— Photographers – Anna Bittner, in Infants Paul DeMarchi we have the opportunity to gather extensive retro- ■ Both craniosynostosis and deform- By Robert Wood, M.D., F.A.C.S., F.A.A.P. spective data. The author presented these findings at Copyright 2013. -
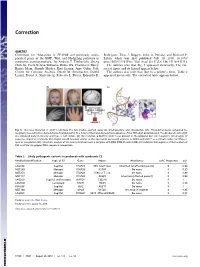
Mutations in TFAP2B and Previously Unimplicated Genes of the BMP, Wnt, and Hedgehog Pathways in Syndromic Craniosynostosis
Correction GENETICS Correction for “Mutations in TFAP2B and previously unim- Rodriguez, Titus J. Boggon, John A. Persing, and Richard P. plicated genes of the BMP, Wnt, and Hedgehog pathways in Lifton, which was first published July 10, 2019; 10.1073/ syndromic craniosynostosis,” by Andrew T. Timberlake, Sheng pnas.1902041116 (Proc. Natl. Acad. Sci. U.S.A. 116,15116–15121). Chih Jin, Carol Nelson-Williams, Robin Wu, Charuta G. Furey, The authors note that Fig. 3 appeared incorrectly. The cor- Barira Islam, Shozeb Haider, Erin Loring, Amy Galm, Yale rected figure and its legend appear below. Center for Genome Analysis, Derek M. Steinbacher, Dawid The authors also note that, due to a printer’s error, Table 2 Larysz, David A. Staffenberg, Roberto L. Flores, Eduardo D. appeared incorrectly. The corrected table appears below. Fig. 3. De novo mutation in SOX11.(A) Note the full cheeks, everted lower lip, brachydactyly, and clinodactyly (37). Three-dimensional computed to- mography reconstruction demonstrates R lambdoid CS. The X-ray of the hand demonstrates absence of the fifth digit distal phalanx. The proband’s sixth digit was removed early in infancy and thus, is not shown. (B) The mutation p.R64H in SOX11 was present in the proband but not in parents. (C) Analysis of sequence alignment indicates that Arg64 should function similar to the equivalent conserved arginine in SOX4 and SOX17 as a critical residue for DNA se- quence recognition (25). Structural analysis of the nearly identical Sox4 in complex with DNA (PDB ID code 3U2B) (25) indicates that arginine at the location of R64 is critical for proper DNA sequence recognition.