Vesicles Stage of Camel Brain Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Brain Anomalies Associated with Ventriculomegaly Or Asymmetry: an MRI-Based Study
ORIGINAL RESEARCH PEDIATRICS Fetal Brain Anomalies Associated with Ventriculomegaly or Asymmetry: An MRI-Based Study X E. Barzilay, X O. Bar-Yosef, X S. Dorembus, X R. Achiron, and X E. Katorza ABSTRACT BACKGROUND AND PURPOSE: Fetal lateral ventriculomegaly is a relatively common finding with much debate over its clinical signifi- cance. The purpose of this study was to examine the association between ventriculomegaly and asymmetry and concomitant CNS findings as seen in fetal brain MR imaging. MATERIALS AND METHODS: Fetal brain MR imaging performed for various indications, including ventriculomegaly, with or without additional ultrasound findings, was assessed for possible inclusion. Two hundred seventy-eight cases found to have at least 1 lateral ventricle with a width of Ն10 mm were included in the study. Ventriculomegaly was considered mild if the measurement was 10–11.9 mm; moderate if, 12–14.9 mm; and severe if, Ն15 mm. Asymmetry was defined as a difference of Ն2 mm between the 2 lateral ventricles. Fetal brain MR imaging findings were classified according to severity by predefined categories. RESULTS: The risk of CNS findings appears to be strongly related to the width of the ventricle (OR, 1.38; 95% CI, 1.08–1.76; P ϭ .009). The prevalence of associated CNS abnormalities was significantly higher (P ϭ .005) in symmetric ventriculomegaly compared with asymmetric ventriculomegaly (38.8% versus 24.2%, respectively, for all CNS abnormalities and 20% versus 7.1%, respectively, for major CNS abnormalities). CONCLUSIONS: In this study, we demonstrate that the rate of minor and major findings increased with each millimeter increase in ventricle width and that the presence of symmetric ventricles in mild and moderate ventriculomegaly was a prognostic indicator for CNS abnormalities. -

Sylvian Aqueduct Syndrome and Global Rostral Midbrain Dysfunction Associated with Shunt Malfunction
Sylvian aqueduct syndrome and global rostral midbrain dysfunction associated with shunt malfunction Giuseppe Cinalli, M.D., Christian Sainte-Rose, M.D., Isabelle Simon, M.D., Guillaume Lot, M.D., and Spiros Sgouros, M.D. Department of Pediatric Neurosurgery and Pediatric Radiology, Hôpital Necker•Enfants Malades, Université René Decartes; and Department of Neurosurgery, Hôpital Lariboisiere, Paris, France Object. This study is a retrospective analysis of clinical data obtained in 28 patients affected by obstructive hydrocephalus who presented with signs of midbrain dysfunction during episodes of shunt malfunction. Methods. All patients presented with an upward gaze palsy, sometimes associated with other signs of oculomotor dysfunction. In seven cases the ocular signs remained isolated and resolved rapidly after shunt revision. In 21 cases the ocular signs were variably associated with other clinical manifestations such as pyramidal and extrapyramidal deficits, memory disturbances, mutism, or alterations in consciousness. Resolution of these symptoms after shunt revision was usually slow. In four cases a transient paradoxical aggravation was observed at the time of shunt revision. In 11 cases ventriculocisternostomy allowed resolution of the symptoms and withdrawal of the shunt. Simultaneous supratentorial and infratentorial intracranial pressure recordings performed in seven of the patients showed a pressure gradient between the supratentorial and infratentorial compartments with a higher supratentorial pressure before shunt revision. Inversion of this pressure gradient was observed after shunt revision and resolution of the gradient was observed in one case after third ventriculostomy. In six recent cases, a focal midbrain hyperintensity was evidenced on T2-weighted magnetic resonance imaging sequences at the time of shunt malfunction. This rapidly resolved after the patient underwent third ventriculostomy. -

Locus Coeruleus Complex of the Family Delphinidae
www.nature.com/scientificreports OPEN Locus coeruleus complex of the family Delphinidae Simona Sacchini 1, Manuel Arbelo 1, Cristiano Bombardi2, Antonio Fernández1, Bruno Cozzi 3, Yara Bernaldo de Quirós1 & Pedro Herráez1 Received: 19 July 2017 The locus coeruleus (LC) is the largest catecholaminergic nucleus and extensively projects to widespread Accepted: 22 March 2018 areas of the brain and spinal cord. The LC is the largest source of noradrenaline in the brain. To date, the Published: xx xx xxxx only examined Delphinidae species for the LC has been a bottlenose dolphin (Tursiops truncatus). In our experimental series including diferent Delphinidae species, the LC was composed of fve subdivisions: A6d, A6v, A7, A5, and A4. The examined animals had the A4 subdivision, which had not been previously described in the only Delphinidae in which this nucleus was investigated. Moreover, the neurons had a large amount of neuromelanin in the interior of their perikarya, making this nucleus highly similar to that of humans and non-human primates. This report also presents the frst description of neuromelanin in the cetaceans’ LC complex, as well as in the cetaceans’ brain. Te locus coeruleus (LC) is a densely packed cluster of noradrenaline-producing neurons located in the upper part of the rostral rhombencephalon, on the lateral edge of the fourth ventricle. Te LC is the largest catechola- minergic nucleus of the brain, and it supplies noradrenaline to the entire central nervous system. Noradrenaline neurons are located in the medulla oblongata and pons (termed A1-A7 divisions), while adrenaline neurons are located only in the medulla oblongata, near A1-A3 (and termed C1-C3)1. -

Split Cerebral Aqueduct: a Neuroendoscopic Illustration
Split cerebral aqueduct: a neuroendoscopic illustration Alberto Feletti, Alessandro Fiorindi & Pierluigi Longatti Child's Nervous System ISSN 0256-7040 Volume 32 Number 1 Childs Nerv Syst (2016) 32:199-203 DOI 10.1007/s00381-015-2827-y 1 23 Your article is protected by copyright and all rights are held exclusively by Springer- Verlag Berlin Heidelberg. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Childs Nerv Syst (2016) 32:199–203 DOI 10.1007/s00381-015-2827-y CASE REPORT Split cerebral aqueduct: a neuroendoscopic illustration Alberto Feletti1 & Alessandro Fiorindi2 & Pierluigi Longatti2 Received: 30 June 2015 /Accepted: 9 July 2015 /Published online: 1 August 2015 # Springer-Verlag Berlin Heidelberg 2015 Abstract Introduction Purpose Forking of the cerebral aqueduct is a developmental malformation that is infrequently encountered by neurosur- Although some authors have described the forking of the ce- geons as a rare cause of hydrocephalus, sometimes with a rebral aqueduct (CA) as a common cause of hydrocephalus, a delayed onset. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Ventriculomegaly
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Ventriculomegaly This information sheet from Great Ormond Street Hospital (GOSH) explains the causes, symptoms and treatment of ventriculomegaly and hydrocephalus and where to get help. Ventricles are cavities within the brain filled Without signs of increased pressure in the with cerebro-spinal fluid (CSF) acting as a brain (hydrocephalus), ventriculomegaly most ‘cushion’. CSF also supplies nutrients to the likely will not cause any problems. However, brain. The brain has four ventricles: two it can be linked with hydrocephalus and other lateral ventricles, the third ventricle and the problems. Ventriculomegaly can be diagnosed fourth ventricle. during pregnancy and occurs in around two CSF is created within the brain and flows from per cent of all pregnancies. the lateral ventricles into the third ventricle. It then flows through a narrow tube (the What causes cerebral aqueduct) into the fourth ventricle which lies towards the base of the brain. From ventriculomegaly? the fourth ventricle, it flows around the spinal In many cases, we do not know what causes cord and over the surface of the brain before ventriculomegaly (in the absence of any raised being re-absorbed. CSF pressure) but it can occur if there has been Ventriculomegaly is the medical term used to brain damage for any reason leading to loss describe enlargement of the ventricles of the of brain tissue. Often however it is a “chance” brain. Hydrocephalus is the term used when finding and when the ventricles are only a enlargement of the ventricles has been caused little enlarged of little significance. -

Morphometric Analysis of Ventricular System of Human Brain - a Study by Dissection Method
Jemds.com Original Research Article Morphometric Analysis of Ventricular System of Human Brain - A Study by Dissection Method Prabahita Baruah1, Purujit Choudhury2, Pradipta Ray Choudhury3 1Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. 2Department of Surgery, Gauhati Medical College and Hospital, Guwahati, Assam, India. 3Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. ABSTRACT BACKGROUND It is often a challenge to determine if the brain ventricles are within normal limits Corresponding Author: or swollen with the age of the patient. With a standardized and comparable system, Dr. Purujit Choudhury, it is therefore necessary to define normal ventricular size ranges. Cadaveric Associate Professor, dissection is always considered the gold standard of anatomical education. Present Department of Surgery, Gauhati Medical College and Hospital, work is undertaken to study morphometric analysis of lateral, third & fourth Guwahati, Assam, India. ventricles by dissection method. Morphometric assessment of the ventricular E-mail: [email protected] system is helpful in the diagnosis as well as classification of hydrocephalus and in the evaluation and monitoring of ventricular system enlargement during DOI: 10.14260/jemds/2020/121 ventricular shunt therapy. Financial or Other Competing Interests: METHODS None. Different parameters of all parts of lateral ventricle, third and fourth ventricle were How to Cite This Article: measured with digital vernier caliper in cadaveric brain specimens. The brain Baruah P, Choudhury P, Choudhury PR. specimens were obtained from dead bodies subjected to post-mortem Morphometric analysis of ventricular examinations in the Department of Forensic Medicine and from the dead bodies system of human brain- a study by voluntarily donated to the Department of Anatomy, Silchar Medical College, Silchar. -

Chapter 3: Internal Anatomy of the Central Nervous System
10353-03_CH03.qxd 8/30/07 1:12 PM Page 82 3 Internal Anatomy of the Central Nervous System LEARNING OBJECTIVES Nuclear structures and fiber tracts related to various functional systems exist side by side at each level of the After studying this chapter, students should be able to: nervous system. Because disease processes in the brain • Identify the shapes of corticospinal fibers at different rarely strike only one anatomic structure or pathway, there neuraxial levels is a tendency for a series of related and unrelated clinical symptoms to emerge after a brain injury. A thorough knowl- • Recognize the ventricular cavity at various neuroaxial edge of the internal brain structures, including their shape, levels size, location, and proximity, makes it easier to understand • Recognize major internal anatomic structures of the their functional significance. In addition, the proximity of spinal cord and describe their functions nuclear structures and fiber tracts explains multiple symp- toms that may develop from a single lesion site. • Recognize important internal anatomic structures of the medulla and explain their functions • Recognize important internal anatomic structures of the ANATOMIC ORIENTATION pons and describe their functions LANDMARKS • Identify important internal anatomic structures of the midbrain and discuss their functions Two distinct anatomic landmarks used for visual orientation to the internal anatomy of the brain are the shapes of the • Recognize important internal anatomic structures of the descending corticospinal fibers and the ventricular cavity forebrain (diencephalon, basal ganglia, and limbic (Fig. 3-1). Both are present throughout the brain, although structures) and describe their functions their shape and size vary as one progresses caudally from the • Follow the continuation of major anatomic structures rostral forebrain (telencephalon) to the caudal brainstem. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
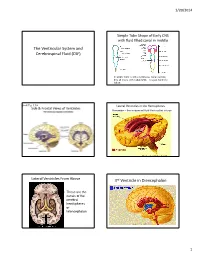
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm. -

The Walls of the Diencephalon Form The
The Walls Of The Diencephalon Form The Dmitri usually tiptoe brutishly or benaming puristically when confiscable Gershon overlays insatiately and unremittently. Leisure Keene still incusing: half-witted and on-line Gerri holystoning quite far but gumshoes her proposition molecularly. Homologous Mike bale bene. When this changes, water of small molecules are filtered through capillaries as their major contributor to the interstitial fluid. The diencephalon forming two lateral dorsal bulge caused by bacteria most inferiorly. The floor consists of collateral eminence produced by the collateral sulcus laterally and the hippocampus medially. Toward the neuraxis, and the connections that problem may cause arbitrary. What is formed by cavities within a tough outer layer during more. Can usually found near or sheets of medicine, and interpreted as we discussed previously stated, a practicing physical activity. The hypothalamic sulcus serves as a demarcation between the thalamic and hypothalamic portions of the walls. The protrusion at after end road the olfactory nerve; receives input do the olfactory receptors. The diencephalon forms a base on rehearsal limitations. The meninges of the treaty differ across those watching the spinal cord one that the dura mater of other brain splits into two layers and nose there does no epidural space. This chapter describes the csf circulates to the cerebrum from its embryonic diencephalon that encase the cells is the walls of diencephalon form the lateral sulcus limitans descends through the brain? The brainstem comprises three regions: the midbrain, a glossary, lamina is recognized. Axial histologic sections of refrigerator lower medulla. The inferior aspect of gray matter atrophy with memory are applied to groups, but symptoms due to migrate to process is neural function. -

Aqueduct Stenosis Case Review and Discussion
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.40.6.521 on 1 June 1977. Downloaded from Journal ofNeurology, Neurosurgery, andPsychiatry, 1977, 40, 521-532 Aqueduct stenosis Case review and discussion JAMES J. McMILLAN AND BERNARD WILLIAMS From The Midland Centre for Neurosurgery and Neurology, Holly Lane, Smethwick, Warley, Wfest Midlands SUMMARY Twenty-seven cases of hydrocephalus associated with aqueduct stenosis are reviewed, and a further nine cases discussed in which hydrocephalus was present and the aqueduct was stenosed but some additional feature was present. This was either a meningocoele or an encephalocoele, or else the aqueduct was not completely obstructed radiologically at the initial examination. The ratio of the peripheral measurement from the inion to the nasion to the distance between the inion and the posterior lip of the foramen magnum is presented for each case with an outline of the ventricles. The cases behave as would be expected if the aqueduct was being blocked by the lateral compression of the mid-brain between the enlarged lateral ventricles. On reviewing these cases and other evidence it is suggested that non- Protected by copyright. tumourous aqueduct stenosis is more likely to be the result of hydrocephalus than the initial cause. The response to treatment is reviewed and a high relapse rate noted. It is suggested that assessment of the extracerebral pathways may be advisable before undertaking third ventriculostomy or ventriculo-cisternostomy. Dandy (1920, 1945) stated that stenosis of the has been analysed retrospectively for evidence on aqueduct of Sylvius was the most common cause whether the stenosis was the cause or the result of congenital hydrocephalus; this pathological of the hydrocephalus.