Vagus Nerve Stimulation and Modulation of the Locus Coeruleus in a Preclinical Model for Multiple Sclerosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Brain Anomalies Associated with Ventriculomegaly Or Asymmetry: an MRI-Based Study
ORIGINAL RESEARCH PEDIATRICS Fetal Brain Anomalies Associated with Ventriculomegaly or Asymmetry: An MRI-Based Study X E. Barzilay, X O. Bar-Yosef, X S. Dorembus, X R. Achiron, and X E. Katorza ABSTRACT BACKGROUND AND PURPOSE: Fetal lateral ventriculomegaly is a relatively common finding with much debate over its clinical signifi- cance. The purpose of this study was to examine the association between ventriculomegaly and asymmetry and concomitant CNS findings as seen in fetal brain MR imaging. MATERIALS AND METHODS: Fetal brain MR imaging performed for various indications, including ventriculomegaly, with or without additional ultrasound findings, was assessed for possible inclusion. Two hundred seventy-eight cases found to have at least 1 lateral ventricle with a width of Ն10 mm were included in the study. Ventriculomegaly was considered mild if the measurement was 10–11.9 mm; moderate if, 12–14.9 mm; and severe if, Ն15 mm. Asymmetry was defined as a difference of Ն2 mm between the 2 lateral ventricles. Fetal brain MR imaging findings were classified according to severity by predefined categories. RESULTS: The risk of CNS findings appears to be strongly related to the width of the ventricle (OR, 1.38; 95% CI, 1.08–1.76; P ϭ .009). The prevalence of associated CNS abnormalities was significantly higher (P ϭ .005) in symmetric ventriculomegaly compared with asymmetric ventriculomegaly (38.8% versus 24.2%, respectively, for all CNS abnormalities and 20% versus 7.1%, respectively, for major CNS abnormalities). CONCLUSIONS: In this study, we demonstrate that the rate of minor and major findings increased with each millimeter increase in ventricle width and that the presence of symmetric ventricles in mild and moderate ventriculomegaly was a prognostic indicator for CNS abnormalities. -

Locus Coeruleus Complex of the Family Delphinidae
www.nature.com/scientificreports OPEN Locus coeruleus complex of the family Delphinidae Simona Sacchini 1, Manuel Arbelo 1, Cristiano Bombardi2, Antonio Fernández1, Bruno Cozzi 3, Yara Bernaldo de Quirós1 & Pedro Herráez1 Received: 19 July 2017 The locus coeruleus (LC) is the largest catecholaminergic nucleus and extensively projects to widespread Accepted: 22 March 2018 areas of the brain and spinal cord. The LC is the largest source of noradrenaline in the brain. To date, the Published: xx xx xxxx only examined Delphinidae species for the LC has been a bottlenose dolphin (Tursiops truncatus). In our experimental series including diferent Delphinidae species, the LC was composed of fve subdivisions: A6d, A6v, A7, A5, and A4. The examined animals had the A4 subdivision, which had not been previously described in the only Delphinidae in which this nucleus was investigated. Moreover, the neurons had a large amount of neuromelanin in the interior of their perikarya, making this nucleus highly similar to that of humans and non-human primates. This report also presents the frst description of neuromelanin in the cetaceans’ LC complex, as well as in the cetaceans’ brain. Te locus coeruleus (LC) is a densely packed cluster of noradrenaline-producing neurons located in the upper part of the rostral rhombencephalon, on the lateral edge of the fourth ventricle. Te LC is the largest catechola- minergic nucleus of the brain, and it supplies noradrenaline to the entire central nervous system. Noradrenaline neurons are located in the medulla oblongata and pons (termed A1-A7 divisions), while adrenaline neurons are located only in the medulla oblongata, near A1-A3 (and termed C1-C3)1. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Ventriculomegaly
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Ventriculomegaly This information sheet from Great Ormond Street Hospital (GOSH) explains the causes, symptoms and treatment of ventriculomegaly and hydrocephalus and where to get help. Ventricles are cavities within the brain filled Without signs of increased pressure in the with cerebro-spinal fluid (CSF) acting as a brain (hydrocephalus), ventriculomegaly most ‘cushion’. CSF also supplies nutrients to the likely will not cause any problems. However, brain. The brain has four ventricles: two it can be linked with hydrocephalus and other lateral ventricles, the third ventricle and the problems. Ventriculomegaly can be diagnosed fourth ventricle. during pregnancy and occurs in around two CSF is created within the brain and flows from per cent of all pregnancies. the lateral ventricles into the third ventricle. It then flows through a narrow tube (the What causes cerebral aqueduct) into the fourth ventricle which lies towards the base of the brain. From ventriculomegaly? the fourth ventricle, it flows around the spinal In many cases, we do not know what causes cord and over the surface of the brain before ventriculomegaly (in the absence of any raised being re-absorbed. CSF pressure) but it can occur if there has been Ventriculomegaly is the medical term used to brain damage for any reason leading to loss describe enlargement of the ventricles of the of brain tissue. Often however it is a “chance” brain. Hydrocephalus is the term used when finding and when the ventricles are only a enlargement of the ventricles has been caused little enlarged of little significance. -

Morphometric Analysis of Ventricular System of Human Brain - a Study by Dissection Method
Jemds.com Original Research Article Morphometric Analysis of Ventricular System of Human Brain - A Study by Dissection Method Prabahita Baruah1, Purujit Choudhury2, Pradipta Ray Choudhury3 1Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. 2Department of Surgery, Gauhati Medical College and Hospital, Guwahati, Assam, India. 3Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. ABSTRACT BACKGROUND It is often a challenge to determine if the brain ventricles are within normal limits Corresponding Author: or swollen with the age of the patient. With a standardized and comparable system, Dr. Purujit Choudhury, it is therefore necessary to define normal ventricular size ranges. Cadaveric Associate Professor, dissection is always considered the gold standard of anatomical education. Present Department of Surgery, Gauhati Medical College and Hospital, work is undertaken to study morphometric analysis of lateral, third & fourth Guwahati, Assam, India. ventricles by dissection method. Morphometric assessment of the ventricular E-mail: [email protected] system is helpful in the diagnosis as well as classification of hydrocephalus and in the evaluation and monitoring of ventricular system enlargement during DOI: 10.14260/jemds/2020/121 ventricular shunt therapy. Financial or Other Competing Interests: METHODS None. Different parameters of all parts of lateral ventricle, third and fourth ventricle were How to Cite This Article: measured with digital vernier caliper in cadaveric brain specimens. The brain Baruah P, Choudhury P, Choudhury PR. specimens were obtained from dead bodies subjected to post-mortem Morphometric analysis of ventricular examinations in the Department of Forensic Medicine and from the dead bodies system of human brain- a study by voluntarily donated to the Department of Anatomy, Silchar Medical College, Silchar. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
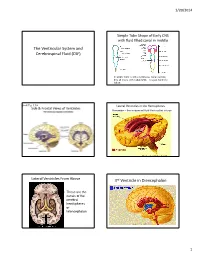
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm. -

The Lateral Ventricles: a Detailed Review of Anatomy, Development, and Anatomic Variations
REVIEW ARTICLE The Lateral Ventricles: A Detailed Review of Anatomy, Development, and Anatomic Variations C.L. Scelsi, T.A. Rahim, J.A. Morris, G.J. Kramer, B.C. Gilbert, and S.E. Forseen ABSTRACT SUMMARY: The cerebral ventricles have been studied since the fourth century BC and were originally thought to harbor the soul and higher executive functions. During the infancy of neuroradiology, alterations to the ventricular shape and position on pneumo- encephalography and ventriculography were signs of mass effect or volume loss. However, in the current era of high-resolution cross-sectional imaging, variation in ventricular anatomy is more easily detectable and its clinical significance is still being investi- gated. Interpreting radiologists must be aware of anatomic variations of the ventricular system to prevent mistaking normal variants for pathology. We will review of the anatomy and development of the lateral ventricles and discuss several ventricular variations. he cerebral ventricles were the center of attention among phi- ventricular size and morphology but have been studied exten- Tlosophers, priests, anatomists, and physicians as far back as sively and will be left out of this review. Aristotle in the fourth century BC.1 They were originally thought to harbor the soul and “vital” spirits responsible for higher func- ANATOMY tions. After the influence of Christianity and the Renaissance, the Lateral Ventricles ventricles were conceptualized as 3 cavities where common sense, The lateral ventricles are paired C-shaped structures comprising creative imagination, and memory were individually allocated. It a body and atrium along with 3 projections into the frontal, tem- was not until the 16th century that anatomists Andreas Vesalius poral, and occipital lobes, termed “horns.” The lateral ventricles and Constanzo Varolio identified ventricles as being filled with communicate with the third ventricle through the interventricu- 2 CSF. -

DEVELOPMENT of CEREBRUM & CEREBELLUM
DEVELOPMENT of CEREBRUM & CEREBELLUM Color Code: ● Important ● Doctors Notes ● Extra explanation OBJECTIVES: ● Describe the formation of the neural tube. ● List the 3 brain vesicles and their derivatives. ● Describe the brain flexures. ● Describe briefly the development of the cerebrum. ● Describe briefly the development of the cerebellum. Introduction: By the beginning of the 3rd week of development, three germ cell layers become established. Ectoderm, Mesoderm and Endoderm. Early Development: During the middle of the 3rd week(16-17 days)the dorsal midline ectoderm undergoes thickening to form the neural plate (neuroectoderm). ● The 2 margins of the plate elevate, forming neural folds ● A longitudinal, midline depression, called the neural groove is formed. ● The 2 neural folds approximate then fuse together, thus sealing the neural groove and creating the neural tube. (complete close). ● Formation of neural tube is completed by the middle of 4th week. Brain develops from cranial 1\3 of neural tube Neural Tube Development: ﺗﻧﻘﺳم ﻣرﺣﻠﺔ ﺗﻛون إﻟﻰ ﻣرﺣﻠﺗﯾن Three- primary brain vesicles stage five secondary brain vesicles stage (end of 4th week) (28 days) (5th week) Neural tube upper end dilates and shows 3 vesicles This 3 vesicles are: (from up to down) Prosencephalon 1. Telencephalon divides into: 1. Prosencephalon (Forebrain) 2. Diencephalon 2. Mesencephalon (Midbrain) Mesencephalon Mesencephalon 3. Rhombencephalon (Hindbrain) Rhombencephalon 1. Metencephalon divides into: 2. Myelencephalon Derivatives of Brain Vesicles: important Primary Brain Vesicles Secondary Brain Vesicles Derivatives In Mature Brain 1. Two telencephalon Cerebral hemisphere Prosencephalon (forebrain) 2.. Diencephalon thalamus Mesencephalon (midbrain) mesencephalon midbrain 1. metencephalon Pons Cerebellum Rhombencephalon (hindbrain) 2. myelencephalon Medulla oblongata By the 4th week, The neural tube grows rapidly and faster than cranial cavity. -

Embryology Team
Embryology team Development of cerebrum and cerebellum Team members 1- Lama Alhwairikh 1-Nawaf Modahi 2- Norah AlRefayi 2- Abdulrahman Ahmed Al-Kadhaib 3- Sara Alkhelb 3- Khalid Al-Own 4-Abdulrahman Al-Khelaif Student Guide: 1- The notes, which are written by the team, are in Blue . 2- Everything written in Red is important. • By the beginning of the 3rd week of development, three germ cell layers become established, 1 ectoderm, mesoderm and endoderm. • During the middle of the 3rd week, the dorsal midline ectoderm undergoes thickening to form the 2 neural plate. • The margins of the plate become elevated, forming 3 neural folds. • A longitudinal, midline depression, called the 4 neural groove is formed. • The 2 neural folds then fuse together, to form 5 the neural tube. • Formation of the neural tube is completed by 6 the middle of the fourth week of development. The brain vesicle grows and gives 3 dilatations named as: • Prosencephalon • Mesencephalon • Rhombencephalon Telencephalon cerebral hemispheres Forebrain prosencephalon Diencephalon Thalami Midbrain mesencephalon Mesencephalon Midbrain Metencephalon Pones & cerebellum Hindbrain rhombencephalon Myelencephalon Medulla By the end of 4th week the 3 primary vesicles develop (3 vesicles stage) By the 5th week 5 secondary vesicles develop (5 vesicles stage) By 4th week: By the 4th week: The neural tube grows rapidly and bends ventrally with the head fold, producing two flexures: Midbrain (cephalic) flexure: In the region of midbrain. Cervical flexure: between the hind brain & the spinal cord. Later Pontine flexure appears in the hindbrain, in the opposite direction of the cephalic & cervical flexures, resulting in stretching and thinning of the roof of the hindbrain. -

The Formation and Function of the Brain Ventricular System
The Formation and Function of the Brain Ventricular System By Jessica T. Chang B.S. Molecular and Cellular Biology The Johns Hopkins University, 2006 Submitted to the Department of Biology in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology June 2012 © 2012 Jessica T. Chang. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part. Signature of author……………………………………………………………………………………………………………………… Department of Biology May 4, 2012 Certified by…..…………………………………………………………………………………………………………………………….. Hazel L. Sive Professor of Biology Associate Dean, School of Science Thesis Supervisor Accepted by …………………………………………………………..…………………………………………………………………… Robert T. Sauer Salvador E. Luria Professor of Biology Chairman, Graduate Student Committee Chang | 1 Chang | 2 The Formation and Function of the Brain Ventricular System By Jessica T. Chang Submitted to the Department of Biology on April 13, 2012 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology ABSTRACT The brain ventricular system is composed of a highly conserved set of cavities that contain cerebrospinal fluid (CSF), a protein-rich fluid essential for brain function. However, little is known about the function of embryonic CSF (eCSF), or the mechanisms of CSF production, retention, and circulation that regulate brain ventricle shape and size. Here we present data that begins to dissect the mechanisms governing CSF dynamics during zebrafish embryonic development. Our data indicate that the Na,K-ATPase regulates three aspects of brain ventricle development essential for normal function - neuroepithelial formation, permeability, and CSF production. -

Mechanisms of Brain Ventricle Development
Mechanisms of Brain Ventricle Development by Laura Anne Lowery B.S. Biology University of California San Diego, 2000 M.S. Biology University of California San Diego, 2001 Submitted to the Department of Biology in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology June 2008 © 2008 Laura Anne Lowery. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part. Signature of Author…………….…………………………………………………………… Department of Biology May 2008 Certified by…….…………………………………………………………………………… Hazel L. Sive Professor of Biology Associate Dean School of Science Thesis Supervisor Accepted by.………………………………………………………...……………………… Stephen P. Bell Professor of Biology Chairman, Graduate Student Committee 1 2 Mechanisms of Brain Ventricle Formation By Laura Anne Lowery Submitted to the Department of Biology on April 2008 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology ABSTRACT The brain ventricles are a conserved system of fluid-filled cavities within the brain that form during the earliest stages of brain development. Abnormal brain ventricle development has been correlated with neurodevelopmental disorders including hydrocephalus and schizophrenia. The mechanisms which regulate formation of the brain ventricles and the embryonic cerebrospinal fluid are poorly understood. Using the zebrafish, I initiated a study of brain ventricle development to define the genes required for this process. The zebrafish neural tube expands into the forebrain, midbrain, and hindbrain ventricles rapidly, over a four-hour window during mid-somitogenesis. In order to determine the genetic mechanisms that affect brain ventricle development, I studied 17 mutants previously-identified as having embryonic brain morphology defects and identified 3 additional brain ventricle mutants in a retroviral-insertion shelf-screen.