(Basin of Saiss, Morocco) in the Perspective of Industrial Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

William Broadhead Professor of History Thesis Supervisor
Vitruvius on Architecture: A Modem Application and Stability Analysis of Classical Structures by Ana S. Escalante Submitted to the Department of Mechanical Engineering in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in Mechanical Engineering at the Lj Massachusetts Institute of Technology June 2013 0 2013 Ana S. Escalante. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created Signature of Author: F , 2 I- - Department of Mechanical Engineering May 28, 2013 Certified by: William Broadhead Professor of History Thesis Supervisor Accepted by: Annette Hosoi Professor of Mechanical Engineering Undergraduate Officer 1 2 Vitruvius on Architecture: A Modem Application and Stability Analysis of Classical Structures by Ana S. Escalante Submitted to the Department of Mechanical Engineering on June 7, 2013 in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in Mechanical Engineering ABSTRACT Imperial Rome has left numerous legacies, the most well-known being its literature and monuments. Though many monuments, such as the Pantheon, are well-preserved, in cases where little physical evidence remains, historians can often use literary sources to inform reconstruction efforts. For more technical studies of Roman construction, technical literature is rare and the contemporary awareness of such literature even less known. When Vitruvius wrote De architectura,he did not intend for it to be a manual for instruction but rather a central source of general architectural knowledge. Directly aimed at architects, contractors, and other individuals involved in the design and construction of buildings, De architecturaprovides insight into contemporary technical knowledge. -

A Mathematician's Lament
A Mathematician’s Lament by Paul Lockhart musician wakes from a terrible nightmare. In his dream he finds himself in a society where A music education has been made mandatory. “We are helping our students become more competitive in an increasingly sound-filled world.” Educators, school systems, and the state are put in charge of this vital project. Studies are commissioned, committees are formed, and decisions are made— all without the advice or participation of a single working musician or composer. Since musicians are known to set down their ideas in the form of sheet music, these curious black dots and lines must constitute the “language of music.” It is imperative that students become fluent in this language if they are to attain any degree of musical competence; indeed, it would be ludicrous to expect a child to sing a song or play an instrument without having a thorough grounding in music notation and theory. Playing and listening to music, let alone composing an original piece, are considered very advanced topics and are generally put off until college, and more often graduate school. As for the primary and secondary schools, their mission is to train students to use this language— to jiggle symbols around according to a fixed set of rules: “Music class is where we take out our staff paper, our teacher puts some notes on the board, and we copy them or transpose them into a different key. We have to make sure to get the clefs and key signatures right, and our teacher is very picky about making sure we fill in our quarter-notes completely. -

An Introduction to the Mandelbrot Set
An introduction to the Mandelbrot set Bastian Fredriksson January 2015 1 Purpose and content The purpose of this paper is to introduce the reader to the very useful subject of fractals. We will focus on the Mandelbrot set and the related Julia sets. I will show some ways of visualising these sets and how to make a program that renders them. Finally, I will explain a key exchange algorithm based on what we have learnt. 2 Introduction The Mandelbrot set and the Julia sets are sets of points in the complex plane. Julia sets were first studied by the French mathematicians Pierre Fatou and Gaston Julia in the early 20th century. However, at this point in time there were no computers, and this made it practically impossible to study the structure of the set more closely, since large amount of computational power was needed. Their discoveries was left in the dark until 1961, when a Jewish-Polish math- ematician named Benoit Mandelbrot began his research at IBM. His task was to reduce the white noise that disturbed the transmission on telephony lines [3]. It seemed like the noise came in bursts, sometimes there were a lot of distur- bance, and sometimes there was no disturbance at all. Also, if you examined a period of time with a lot of noise-problems, you could still find periods without noise [4]. Could it be possible to come up with a model that explains when there is noise or not? Mandelbrot took a quite radical approach to the problem at hand, and chose to visualise the data. -

Does Mathematical Beauty Pose Problems for Naturalism?1
Does Mathematical Beauty Pose Problems for Naturalism?1 Russell W. Howell Professor of Mathematics Westmont College, Santa Barbara, CA [email protected] Numerous events occurred in 1960 whose eects could hardly have been predicted at the time: several African Americans staged a sit-in at a Greensboro lunch counter, the Soviet Union shot down Gary Powers while he was ying a U2 spy plane, the US FDA approved the use of the rst oral contraceptive, AT&T led with the Federal Communications Commission for permission to launch an experimental communications satellite, and four Presidential debates between John Kennedy and Richard Nixon aired on national television. Less well known was the publication of a paper by the physicist Eugene Wigner. Ap- pearing in Communications in Pure and Applied Mathematics, a journal certainly not widely read by the general public, it bore the mysterious title The Unreasonable Eectiveness of Mathematics in the Natural Sciences. (Wigner, 1960) Like our cultural examples of the 1960's, it has had eects beyond what most people would have imagined. Our purpose here is to tease out some strains of an important question that has emerged from Wigner's work. Wigner begins with a story about two friends who were discussing their jobs. One of them, a statistician, was working on population trends. He mentioned a paper he had produced, which contained the Gaussian distribution equation near the beginning. The statistician attempted to explain the meaning it, as well as other mathematical symbols. His friend was a bit perplexed, and was not quite sure whether the statistician was pulling his leg. -

Bridge Design Manual (BDM), 2006
Bridge Design Manual June 2006 WWW.SCDOT.ORG Welcome To The SCDOT Bridge Design Manual Chapter 1 – Bridge Design Section Organization Chapter 2 – Bridge Replacement Project Development Chapter 3 – Procedures for Plan Preparation Chapter 4 – Coordination of Bridge Replacement Projects Chapter 5 – Administrative Policies and Procedures Chapter 6 – Plan Preparation Chapter 7 – Quantity Estimates Chapter 8 – Construction Cost Estimates Chapter 9 – Bid Documents Chapter 10 – [Reserved] Chapter 11 – General Requirements Chapter 12 – Structural Systems and Dimensions Chapter 13 – Loads and Load Factors Chapter 14 – Structural Analysis and Evaluation Chapter 15 – Structural Concrete Chapter 16 – Structural Steel Structures Chapter 17 – Bridge Decks Chapter 18 – Bridge Deck Drainage Chapter 19 – Foundations Chapter 20 – Substructures Chapter 21 – Joints and Bearings Chapter 22 – Highway Bridges Over Railroads Chapter 23 – Bridge Widening and Rehabilitation Chapter 24 – Construction Operations Chapter 25 – Computer Programs Chapter 1 BRIDGE DESIGN SECTION ORGANIZATION SCDOT BRIDGE DESIGN MANUAL April 2006 SCDOT Bridge Design Manual BRIDGE DESIGN SECTION ORGANIZATION Table of Contents Section Page 1.1 BRIDGE DESIGN SECTION...................................................................................1-1 1.1.1 State Bridge Design Engineer..................................................................1-1 1.1.2 Seismic Design and Bridge Design Support Team..................................1-2 1.1.3 Bridge Design Teams...............................................................................1-3 -

Fractal Art(Ists)
FRACTAL ART(ISTS) Marco Abate and Beatrice Possidente 1 Introduction “Fractals are everywhere.” This has been a sort of advertising line for fractal geometry (and for mathematics in general) since the Eighties, when Benoît Mandelbrot discovered the set now known as Mandelbrot set, and brought fractals to the general attention (see, e.g., [1, 2] for the story of his discovery, and [3] for an introduction to fractal geometry). As most good advertising lines, though not completely true, it is not too far from the truth. Possibly they are not exactly everywhere, but fractals are frequent enough to guarantee you will meet at least one as soon as you leave your full-of-straight-lines man-made-only office space and take a short walk in a natural environment. When you start thinking about it, this is not surprising. Fractals are easy to generate: it suffices to repeat over and over (infinitely many times in the mathematical world, but in the real world ten times is often close enough to infinity) the same simple process to create a complicated object; and tinkering with just a few parameters allows to obtain an amazingly wide range of different shapes, that can be easily adapted to specific requirements. It is quite an efficient scheme, and nature loves efficiency; complex structures can be created starting just from simple (but nonlinear!) tools. Coastlines and mountain lines; leaves arrangements and lightning; fractures and clouds; all examples of fractally generated shapes. And fractals are beautiful, there is no denying that. Something in their intricate shapes, in their repeating of similar forms at different scales, resonates deeply into our (fractal-shaped) brain, evokes our aesthetic senses, let us admire infinity easing our painfully perceived finiteness. -

Numerical Anamorphosis: an Artistic Exploration
Numerical Anamorphosis: an Artistic Exploration FRANCESCO DE COMITE and LAURENT GRISONI CRIStAL laboratory University of Sciences of Lille, France [francesco.de-comite/laurent.grisoni]@univ-lille1.fr Anamorphoses have been know for centuries, as distorted images needing —Consider a non-flat geometry: how to compute the image to be to be seen in a mirror from a special point of view in order to see the non- seen on this screen? The computation of distorted images will be distorted image. During Renaissance, they have been associated to mathe- the subject of section 4. matical techniques for drawing such pictures efficiently, on specific mirror —Consider a visual message we want to share: what geometries shapes (in the case of cylindrical or conical mirrors). We can expect in the can we define, that will convey this information? The definition next years a strong interest in such type of images, because of the emergence and computation of distorted three-dimensional objects will be of various contexts and physical supports for image visualization (soft or de- studied in sections 5.1 and 5.2. formable screens, lightmapping, projection of images on dynamic objects, etc...). Solving the numerical problem of anamorphosis in the general case The same questions arose at the time of Renaissance, when belongs to the same class of problems as when the trend is to control im- artists wanted to know how to paint three-dimensional scenes on age deformation as long as image is seen projected on, or reflected by, a flat canvasses, and discovered the laws of perspective. Some of non-planar surface, which can be of arbitrary shape. -

Episode 3: Can Math Be Beautiful? Transcript (Note: This Is the Result Of
Episode 3: Can math be beautiful? Transcript (Note: This is the result of a hybrid between machine learning and human correction, so it may have slight imperfections.) Michael Atiyah: 00:05 We mathematicians and scientists are searching for truth. But beauty is our guiding light that leads us there. Stephen Ornes: 00:14 This is the third episode of Calculated, a podcast collection of stories about people at the intersection of math and art and culture and today we're talking about beauty. Semir Zeki: 00:24 Mathematical beauty is the most extreme form of beauty that is dependent upon culture and learning because you cannot, you cannot invite somebody to your office or to your room and tell them, is this mathematical formula beautiful or not? Unless they're mathematicians, they're not going to understand it. Stephen Ornes: 00:42 People can be beautiful. Music, beautiful. Paintings, buildings, minerals, poetry, sunrises. Those amazing bands of turbulence on Jupiter. Beautiful! But what is beauty anyway? What about math? Can math be beautiful? What does that even mean? And what about people who hate math but know beauty? What's in it for them? Paul Erdos, one of the most prolific mathematicians in all of mathtopia, was once asked to talk about how he found math beautiful. He said, it's like asking why is Beethoven's Ninth Symphony beautiful? If you don't see why, someone can't tell you. I guess that puts it in the same group as irony or obscenity. You can't define it, but you know it when you see it. -
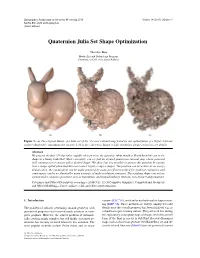
Quaternion Julia Set Shape Optimization
Eurographics Symposium on Geometry Processing 2015 Volume 34 (2015), Number 5 Mirela Ben-Chen and Ligang Liu (Guest Editors) Quaternion Julia Set Shape Optimization Theodore Kim Media Arts and Technology Program University of California, Santa Barbara (a) (b) (c) Figure 1: (a) The original Bunny. (b) Julia set of the 331-root rational map found by our optimization. (c) Highly intricate surface obtained by translating the roots by 1.41 in the z direction. Image is high-resolution; please zoom in to see details. Abstract We present the first 3D algorithm capable of answering the question: what would a Mandelbrot-like set in the shape of a bunny look like? More concretely, can we find an iterated quaternion rational map whose potential field contains an isocontour with a desired shape? We show that it is possible to answer this question by casting it as a shape optimization that discovers novel, highly complex shapes. The problem can be written as an energy minimization, the optimization can be made practical by using an efficient method for gradient evaluation, and convergence can be accelerated by using a variety of multi-resolution strategies. The resulting shapes are not in- variant under common operations such as translation, and instead undergo intricate, non-linear transformations. Categories and Subject Descriptors (according to ACM CCS): I.3.5 [Computer Graphics]: Computational Geometry and Object Modeling—Curve, surface, solid, and object representations 1. Introduction variants [LLC∗10], and similar methods such as hypertextur- ing [EMP∗02]. These methods are widely employed to add The problem of robustly generating smooth geometry with details once the overall geometry has been finalized, e.g. -

Csi2013program and Abstract
XXXVIII CSI 2013 Table of Content TABLE OF CONTENT Table of Content ................................................................................................................. 1 Welcome! .............................................................................................................................. 2 Organising and Scientific Committee ...................................................................... 3 International Advisory Board ....................................................................................... 4 Continuation Committee ................................................................................................ 4 General Information ......................................................................................................... 5 Social Programme ............................................................................................................. 8 Scientific Programme ................................................................................................... 10 Liability ................................................................................................................................ 10 Sponsors and Exhibitors .............................................................................................. 11 Correspondence after the conference ................................................................. 12 Schedule of Events ......................................................................................................... 13 Daily Programme -

Interactive Modeling of Muqarnas
Computational Aesthetics in Graphics, Visualization, and Imaging (2011) D. Cunningham and T. Isenberg (Editors) Interactive Modeling of Muqarnas N. Hamekasi1, F.F. Samavati1 and A. Nasri2 1Department of Computer Science, University of Calgary, Canada 2Department of Computer Science, American University of Beirut Figure 1: Model of an existing building. Abstract Muqarnas is a mesmerizing 3D feature of Islamic architecture that exhibit intricate geometry. Such designs are composed of several basic structures combined in successive layers, producing complicated 3D surfaces. In this paper we propose a new approach for interactive modelling of muqarnas based on their layered structure. As a guidance for the modeling workflow, floor plans are used. We also discuss how to edit the resulting models and how to automatically generate new forms. Categories and Subject Descriptors (according to ACM CCS): I.3.5 [Computer Graphics]: Computational Geometry and Object Modeling—Modeling packages 1. Introduction tations in artistic expression. Consequently, Islamic art has a rich mathematical foundation. Mathematics and art, these two seemingly diverse aspects of humanity, have influenced each other from the early days. Islamic geometric decorations can be categorized into two Over thousands of years, the use of geometric patterns as major categories: two dimensional and three dimensional decorations is well established. Art has evolved and devel- patterns. The first group is the type of ornament used to dec- oped along with humankind, changing not only with time orate flat or curved surfaces, such as domes or walls. A well- but also with cultural and geographical conditions, result- known example of this category is star pattern. The other ing in a diversity of styles. -

The Meaning of the Great Mosque of Cordoba in the Tenth Century Author(S): Nuha N
The Meaning of the Great Mosque of Cordoba in the Tenth Century Author(s): Nuha N. N. Khoury Source: Muqarnas, Vol. 13 (1996), pp. 80-98 Published by: BRILL Stable URL: http://www.jstor.org/stable/1523253 Accessed: 09/10/2009 09:33 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=bap. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. BRILL is collaborating with JSTOR to digitize, preserve and extend access to Muqarnas. http://www.jstor.org NUHA N.N. KHOURY THE MEANING OF THE GREATMOSQUE OF CORDOBA IN THE TENTH CENTURY The Great Mosque of Cordoba is universally recognized however, is morphologically and aesthetically distinct: a as one of the most singular monuments of medieval unique reformulation of preexisting architectural architecture.