2,628,249 United States Patent Office
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cheminformatics for Genome-Scale Metabolic Reconstructions
CHEMINFORMATICS FOR GENOME-SCALE METABOLIC RECONSTRUCTIONS John W. May European Molecular Biology Laboratory European Bioinformatics Institute University of Cambridge Homerton College A thesis submitted for the degree of Doctor of Philosophy June 2014 Declaration This thesis is the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. This dissertation is not substantially the same as any I have submitted for a degree, diploma or other qualification at any other university, and no part has already been, or is currently being submitted for any degree, diploma or other qualification. This dissertation does not exceed the specified length limit of 60,000 words as defined by the Biology Degree Committee. This dissertation has been typeset using LATEX in 11 pt Palatino, one and half spaced, according to the specifications defined by the Board of Graduate Studies and the Biology Degree Committee. June 2014 John W. May to Róisín Acknowledgements This work was carried out in the Cheminformatics and Metabolism Group at the European Bioinformatics Institute (EMBL-EBI). The project was fund- ed by Unilever, the Biotechnology and Biological Sciences Research Coun- cil [BB/I532153/1], and the European Molecular Biology Laboratory. I would like to thank my supervisor, Christoph Steinbeck for his guidance and providing intellectual freedom. I am also thankful to each member of my thesis advisory committee: Gordon James, Julio Saez-Rodriguez, Kiran Patil, and Gos Micklem who gave their time, advice, and guidance. I am thankful to all members of the Cheminformatics and Metabolism Group. -

Partial Characterization of Soil Humic Acids Through Biodegradation1
PARTIAL CHARACTERIZATION OF SOIL HUMIC ACIDS THROUGH BIODEGRADATION1 S. P. A~IATHUR~AND E. A. PAUL Deparln~enlof Soil Science, University of Saskatchewan, Saskaloon, Saskatchezoan Received October 12, 1966 A strain of Pewicillizi?tz freqzientans was successfully employed for partial degradation and characterization of humic acids. Salicyl alcohol and salicylalcle- hyde were detected in culture filtrates of the fungus ~rtilizinghumates under reduced oxygen tension. The enzyme systems iiivolved in the degradation of humic acids were adaptive. The humate-adapted lnyceliirln was capable of metabolizi~~ga number of co~npoundswhich occur in soil as products of degrada- tion of li~nir~,aromatic amino acids, and plant glycosides but not polyphenolic hydrocarbons, resorcinol, and phlorogluci~~ol. Introduction In a previous paper the authors suggested the use of biodegradation for determining the structural features of hurnic acids and reported isolation of a number of organisms capable of utilizing these acids (7). One such isolate, a strain of Penicilliunz frequentans, degraded 32% of huinic acids in replace- ment-shake culture (7). The organisin appeared to use the predominantly aromatic 'core' of humic acids as a source of carbon (7). This paper reports an attempt to characterize a portion of the humic acids through identification of the initial products of their degradation by the penicillium. Materials and Methods Hzimic Acids The 'mobile' humic acids used in these experiments were extracted from the Ap horizon of a Allelfort Orthic Black soil by standard alkali and acid fraction- For personal use only. ation technique (1). These huinic acids were found, by 14C dating, to have a mean residence time of 785 + 50 years (1). -

Pairwise Oxidative Dearomatization and N
PAIRWISE OXIDATIVE DEAROMATIZATION AND N-HYDROXYCARBAMATE DEHYDROGENATION: MOLECULAR COMPLEXITY VIA AN ACYLNITROSO DIELS–ALDER CYCLOADDITION CASCADE INSPIRED BY TETRODOTOXIN Steffen N. Good A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2018 Approved by: Jeffrey S. Johnson Sidney M. Wilkerson-Hill Michel R. Gagné Alexander J. M. Miller Bo Li © 2018 Steffen N. Good ALL RIGHTS RESERVED ii ABSTRACT Steffen N. Good: Development and Application of an Oxidative Dearomatization/Acylnitroso Diels–Alder Cycloaddition Cascade Toward the Total Synthesis of (±)-Tetrodotoxin (Under the direction of Jeffrey S. Johnson) I. Highly Functionalized Tricyclic Oxazinanones via Pairwise Oxidative Dearomatization and N-Hydroxycarbamate Dehydrogenation: Molecular Diversity Inspired by Tetrodotoxin. Benzenoids in principle represent attractive and abundant starting materials for the preparation of substituted cyclohexanes; however, the synthetic tools available for overcoming the considerable aromatic energies inherent to these building blocks limit the available product types. Drawing inspiration from the complex natural product tetrodotoxin, we demonstrate access to heretofore unknown heterotricyclic structures by leveraging oxidative dearomatization of 2-hydroxymethyl phenols with concurrent N- hydroxycarbamate dehydrogenation using a common oxidant. The pairwise-generated, mutually reactive -

Carboxylic Acids
13 Carboxylic Acids The active ingredients in these two nonprescription pain relievers are derivatives of arylpropanoic acids. See Chemical Connections 13A, “From Willow Bark to Aspirin and Beyond.” Inset: A model of (S)-ibuprofen. (Charles D. Winters) KEY QUESTIONS 13.1 What Are Carboxylic Acids? HOW TO 13.2 How Are Carboxylic Acids Named? 13.1 How to Predict the Product of a Fischer 13.3 What Are the Physical Properties of Esterification Carboxylic Acids? 13.2 How to Predict the Product of a B-Decarboxylation 13.4 What Are the Acid–Base Properties of Reaction Carboxylic Acids? 13.5 How Are Carboxyl Groups Reduced? CHEMICAL CONNECTIONS 13.6 What Is Fischer Esterification? 13A From Willow Bark to Aspirin and Beyond 13.7 What Are Acid Chlorides? 13B Esters as Flavoring Agents 13.8 What Is Decarboxylation? 13C Ketone Bodies and Diabetes CARBOXYLIC ACIDS ARE another class of organic compounds containing the carbonyl group. Their occurrence in nature is widespread, and they are important components of foodstuffs such as vinegar, butter, and vegetable oils. The most important chemical property of carboxylic acids is their acidity. Furthermore, carboxylic acids form numerous important derivatives, including es- ters, amides, anhydrides, and acid halides. In this chapter, we study carboxylic acids themselves; in Chapters 14 and 15, we study their derivatives. 457 458 CHAPTER 13 Carboxylic Acids 13.1 What Are Carboxylic Acids? Carboxyl group A J COOH The functional group of a carboxylic acid is a carboxyl group, so named because it is made group. up of a carbonyl group and a hydroxyl group (Section 1.7D). -

Antioxidant, Cytotoxic, and Antimicrobial Activities of Glycyrrhiza Glabra L., Paeonia Lactiflora Pall., and Eriobotrya Japonica (Thunb.) Lindl
Medicines 2019, 6, 43; doi:10.3390/medicines6020043 S1 of S35 Supplementary Materials: Antioxidant, Cytotoxic, and Antimicrobial Activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. Extracts Jun-Xian Zhou, Markus Santhosh Braun, Pille Wetterauer, Bernhard Wetterauer and Michael Wink T r o lo x G a llic a c id F e S O 0 .6 4 1 .5 2 .0 e e c c 0 .4 1 .5 1 .0 e n n c a a n b b a r r b o o r 1 .0 s s o b b 0 .2 s 0 .5 b A A A 0 .5 0 .0 0 .0 0 .0 0 5 1 0 1 5 2 0 2 5 0 5 0 1 0 0 1 5 0 2 0 0 0 1 0 2 0 3 0 4 0 5 0 C o n c e n tr a tio n ( M ) C o n c e n tr a tio n ( M ) C o n c e n tr a tio n ( g /m l) Figure S1. The standard curves in the TEAC, FRAP and Folin-Ciocateu assays shown as absorption vs. concentration. Results are expressed as the mean ± SD from at least three independent experiments. Table S1. Secondary metabolites in Glycyrrhiza glabra. Part Class Plant Secondary Metabolites References Root Glycyrrhizic acid 1-6 Glabric acid 7 Liquoric acid 8 Betulinic acid 9 18α-Glycyrrhetinic acid 2,3,5,10-12 Triterpenes 18β-Glycyrrhetinic acid Ammonium glycyrrhinate 10 Isoglabrolide 13 21α-Hydroxyisoglabrolide 13 Glabrolide 13 11-Deoxyglabrolide 13 Deoxyglabrolide 13 Glycyrrhetol 13 24-Hydroxyliquiritic acid 13 Liquiridiolic acid 13 28-Hydroxygiycyrrhetinic acid 13 18α-Hydroxyglycyrrhetinic acid 13 Olean-11,13(18)-dien-3β-ol-30-oic acid and 3β-acetoxy-30-methyl ester 13 Liquiritic acid 13 Olean-12-en-3β-ol-30-oic acid 13 24-Hydroxyglycyrrhetinic acid 13 11-Deoxyglycyrrhetinic acid 5,13 24-Hydroxy-11-deoxyglycyirhetinic -

Characterization of Diverse Mechanisms of Salicin Degradation in Populus Microbiome Isolates
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2019 Characterization of Diverse Mechanisms of Salicin Degradation in Populus Microbiome Isolates Sanjeev Dahal University of Tennessee, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Dahal, Sanjeev, "Characterization of Diverse Mechanisms of Salicin Degradation in Populus Microbiome Isolates. " PhD diss., University of Tennessee, 2019. https://trace.tennessee.edu/utk_graddiss/5720 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Sanjeev Dahal entitled "Characterization of Diverse Mechanisms of Salicin Degradation in Populus Microbiome Isolates." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Life Sciences. Jennifer Morrell-Falvey, Major Professor We have read this dissertation and recommend its acceptance: Dale Pelletier, Sarah Lebeis, Cong Trinh, Daniel Jacobson Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Characterization of Diverse Mechanisms of Salicin Degradation in Populus Microbiome Isolates A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Sanjeev Dahal December 2019 Dedication I would like to dedicate this dissertation, first and foremost to my family. -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Role of Deposition Temperature and Concentration on the Self-Assembly and Reaction of Organic Molecules at the Solution-Graphite Interface
Role of deposition temperature and concentration on the self-assembly and reaction of organic molecules at the solution-graphite interface von der Fakultät für Naturwissenschaften der Technischen Universität Chemnitz genehmigte Dissertation zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) vorgelegt von M.Sc. Nguyen Doan Chau Yen geboren am 15.05.1983 in Quang Nam, Viet Nam eingereicht am 09. September 2016 Gutachter: Prof. Dr. Michael Hietschold Prof. Dr. Carsten Deibel Tag der Verteidigung: 17. Januar 2017 Bibliographische Beschreibung M.Sc. Nguyen Doan Chau Yen “Role of deposition temperature and concentration on the self-assembly and reaction of organic molecules at the solution-graphite interface” Technische Universität Chemnitz, Fakultät für Naturwissenschaften, Chemnitz Dissertation (in englischer Sprache), September 2016 120 Seiten, 52 Abbildungen, 6 Tabellen Referat Das Hauptthema dieser Dissertation ist die Untersuchung der Selbstorganisation organischer Moleküle an der Flüssig-Fest-Grenzfläche (LSI). Besondere Betonung liegt auf der Kontrolle der Selbstassemblierung durch geeignete Parameter: die Substrattemperatur während der Abscheidung, die Konzentration der gelösten Moleküle, und die chemische Natur der gelösten Stoffe und Lösungsmittel. Die Untersuchungen wurden unter Verwendung der Rastertunnelmikroskopie (STM) durchgeführt. Der erste Schwerpunkt dieser Arbeit ist die systematische Untersuchung der Auswirkung erhöhter Substrattemperatur während der Abscheidung aus der Lösung auf die Selbstorganisation komplexer molekularer Architekturen an der LSI. Diese Untersuchungen wurden mit dem planaren Molekül Trimesinsäure (TMA), sowie dem nicht- planaren Molekül Benzen-1,3,5-triphosphonsäure (BTP) durchgeführt. Es wird gezeigt, dass der Polymorphismus der Adsorbatstrukturen von TMA und BTP durch die Substrattemperatur während der Abscheidung der Moleküle aus der Lösung für verschiedene Lösungsmitteln unterschiedlicher Polarität, wie Phenyloctan, Octansäure und Undecanol, kontrolliert werden kann. -
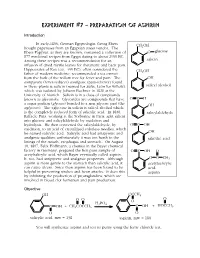
Experiment 7
Experiment #7 – Preparation of Aspirin Introduction In early-1800, German Egyptologist Georg Ebers CH2OH bought papyruses from an Egyptian street vendor. The Ebers Papyrus, as they are known, contained a collection of O glucose 877 medicinal recipes from Egypt dating to about 2500 BC. Among these recipes was a recommendation for an salicin infusion of dried myrtle leaves for rheumatic and back pain. Hippocrates of Kos (ca. 400 BC), often considered the CH2OH father of modern medicine, recommended a tea extract from the bark of the willow tree for fever and pain. The OH antipyretic (fever-reducer) analgesic (pain-reliever) found in these plants is salicin (named for Salix, Latin for willow), salicyl alcohol which was isolated by Johann Buchner in 1828 at the University of Munich. Salicin is in a class of compounds known as glycosides. Glycosides are compounds that have OHC a sugar portion (glycose) bonded to a non-glycose part (the OH aglycone). The aglycone in salicin is salicyl alcohol which is the completely reduced form of salicylic acid. In 1838, salicylaldehyde Raffaele Piria, working at the Sorbonne in Paris, split salicin into glucose and salicylaldehyde by oxidation and hydrolysis. He then converted the salicylaldehyde, by OOHC oxidation, to an acid of crystallized colorless needles, which he named salicylic acid. Salicylic acid had antipyretic and OH analgesic qualities; unfortunately it was too harsh to the salicylic acid linings of the mouth, esophagus and stomach. On August 10, 1897, Felix Hoffmann, a chemist in the Bayer chemical factory in Germany, prepared the first pure sample of OOHC O acetylsalicylic acid, which Bayer eventually called aspirin. -

Annex I: Clinical Evidence Regarding Sensitisation to Individual Fragrance Chemicals and to Natural Extracts
Annex I: Clinical evidence regarding sensitisation to individual fragrance chemicals and to natural extracts Contents Single chemicals ............................................................................. 2 Catalogue of single chemicals evaluated ............................................. 5 Natural extracts / essential oils ........................................................68 Catalogue of natural extracts / essential oils evaluated ........................68 References ....................................................................................99 1 Single chemicals Often, results with the single constituents of the FM I or, yet more rarely, FM II, are presented in one paper. As the main ordering is by allergen, core information on these studies is presented in a tabular format and referenced by a unique acronym in the single sections, to avoid redundancy. Regarding nomenclature, terms which are often not officially an INCI Name but Perfuming Name as listed by CosIng are used. Table 1: Background information on studies reporting results with (all) single constituents of the FM I (amyl cinnamal, cinnamyl alcohol, cinnamal, eugenol, geraniol, hydroxycitronellal, isoeugenol, EVERNIA PRUNASTRI) Reference Country Study period, Comments by Patients reviewers Larsen 2002 c 7 industrial countries Prior to 2002 Test concentrations (1) worldwide n=218 patients with identified as non- known contact allergy irritating in serial to fragrance dilution testing in 20 ingredients healthy volunteers Utrecht 1999 Utrecht, The Netherlands -
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Molecules 2016, 21, 75; doi:10.3390/molecules21010075 S1 of S110 Supplementary Materials: Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs Fei Mao 1, Wei Ni 1, Xiang Xu 1, Hui Wang 1, Jing Wang 1, Min Ji 1 and Jian Li * Table S1. Common names, indications, CAS Registry Numbers and molecular formulas of 6891 approved drugs. Common Name Indication CAS Number Oral Molecular Formula Abacavir Antiviral 136470-78-5 Y C14H18N6O Abafungin Antifungal 129639-79-8 C21H22N4OS Abamectin Component B1a Anthelminithic 65195-55-3 C48H72O14 Abamectin Component B1b Anthelminithic 65195-56-4 C47H70O14 Abanoquil Adrenergic 90402-40-7 C22H25N3O4 Abaperidone Antipsychotic 183849-43-6 C25H25FN2O5 Abecarnil Anxiolytic 111841-85-1 Y C24H24N2O4 Abiraterone Antineoplastic 154229-19-3 Y C24H31NO Abitesartan Antihypertensive 137882-98-5 C26H31N5O3 Ablukast Bronchodilator 96566-25-5 C28H34O8 Abunidazole Antifungal 91017-58-2 C15H19N3O4 Acadesine Cardiotonic 2627-69-2 Y C9H14N4O5 Acamprosate Alcohol Deterrant 77337-76-9 Y C5H11NO4S Acaprazine Nootropic 55485-20-6 Y C15H21Cl2N3O Acarbose Antidiabetic 56180-94-0 Y C25H43NO18 Acebrochol Steroid 514-50-1 C29H48Br2O2 Acebutolol Antihypertensive 37517-30-9 Y C18H28N2O4 Acecainide Antiarrhythmic 32795-44-1 Y C15H23N3O2 Acecarbromal Sedative 77-66-7 Y C9H15BrN2O3 Aceclidine Cholinergic 827-61-2 C9H15NO2 Aceclofenac Antiinflammatory 89796-99-6 Y C16H13Cl2NO4 Acedapsone Antibiotic 77-46-3 C16H16N2O4S Acediasulfone Sodium Antibiotic 80-03-5 C14H14N2O4S Acedoben Nootropic 556-08-1 C9H9NO3 Acefluranol Steroid -
Reference Substances 2018/2019
Reference Substances 2018 / 2019 Reference Substances Reference 2018/2019 Contents | 3 Contents Page Welcome 4 Our Services 5 Reference Substances 6 Index I: Alphabetical List of Reference Substances and Synonyms 156 Index II: Plant-specific Marker Compounds 176 Index III: CAS Registry Numbers 214 Index IV: Substance Classification 224 Our Reference Substance Team 234 Order Information 237 Order Form 238 Prices insert 4 | Welcome Welcome to our new 2018 / 2019 catalogue! PhytoLab proudly presents the new you will also be able to view exemplary Index I contains an alphabetical list of all 2018 / 2019 catalogue of phyproof® certificates of analysis and download substances and their synonyms. It pro- Reference Substances. The seventh edition material safety data sheets (MSDS). vides information which name of a refer- of our catalogue now contains well over ence substance is used in this catalogue 1300 phytochemicals. As part of our We very much hope that our product and guides you directly to the correct mission to be your leading supplier of portfolio meets your expectations. The list page. herbal reference substances PhytoLab of substances will be expanded even has characterized them as primary further in the future, based upon current If you are a planning to analyse a specific reference substances and will supply regulatory requirements and new scientific plant please look for the botanical them together with the comprehensive developments. The most recent information name in Index II. It will inform you about certificates of analysis you are familiar will always be available on our web site. common marker compounds for this herb. with.